Continuous monitoring after surgery is essential to catch early signs of complications when risk of mortality is at its highest. However, more monitoring means more alarms, many potentially false, which raises risk of alarm fatigue among clinicians. A new study indicates that technology with customizable alarm thresholds can provide the continuous postoperative care patients need with fewer false alarms than current methods.
The COSMOS study
Mobile monitoring solutions are intended to detect deterioration as it's happening while giving patients the freedom to move about the hospital. With appropriate alarm thresholds, a mobile monitoring solution provides continuous feedback without overwhelming clinicians with false alarms.
To determine whether GE HealthCare's Portrait™ Mobile monitoring solution met these objectives, the Cleveland Clinic conducted a pilot study, called COSMOS (Continuous Ward Monitoring with the GE HealthCare Portrait Mobile Monitoring Solution), funded by GE HealthCare. The purpose of the study was threefold:
- To evaluate patient tolerance of GE HealthCare's Portrait Mobile monitoring solution
- To evaluate appropriate alarm thresholds
- To assess clinical utility
The pilot study measured the frequency of respiratory events as well as SpO2 and pulse rate abnormalities in 100 patients in a surgical ward. The results were intended to help Cleveland Clinic identify meaningful vital sign abnormalities, as well as optimize alarm settings to mitigate false alerts.
The study team evaluated alarms for low SpO2, high and low pulse rate, and high and low respiratory rate. Over the course of 253 days, the device produced 1,329 alarms. Predictive analytics software used in the study helped the team evaluate the interaction of physiologic data, duration of alarm delays, and alarm thresholds at various device configurations.
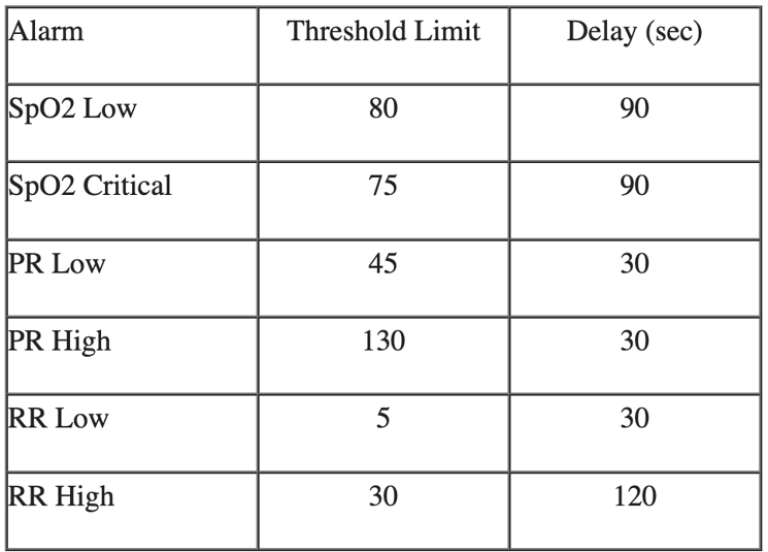
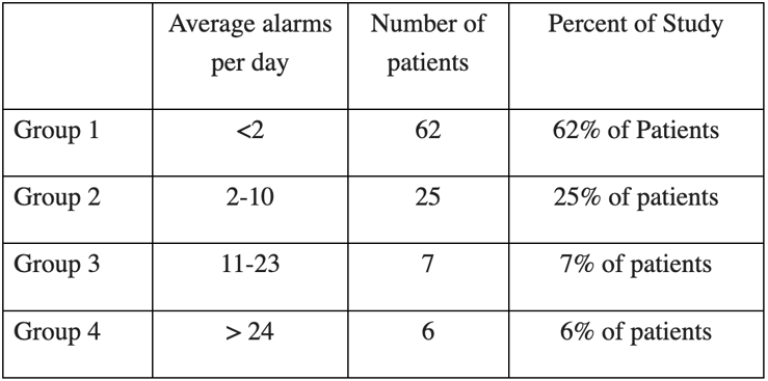
At the designated alarm thresholds, key figures from these results include:
- 62% of patients activated fewer than two alarms per day
- 25% activated 2-10 alarms per day
- 7% activated 11-23 alarms per day
- 6% activated more than 24 alarms per day
Six outlier patients accounted for 46% of the total alarms, compared to just 54% across the remaining 94 patients. After adjusting alarm thresholds for the 6% outliers, the study results showed the Portrait Mobile monitoring solution produced only one alarm every eight hours.
"A process that allows customizable alarm configuration on a per-patient basis seems likely to improve usability," the study authors concluded. "Further development of an alarm burden tool based on alarm event counts that models predicted alarm burden may guide setting optimal thresholds and delays in various units."1
"With Portrait Mobile, medical-surgical teams can optimize alarm settings for their specific unit needs," says Cory Stahl, global marketing manager for GE HealthCare. "They're not going to get the nuisance alarms, false alarms, and transient alarms that they may get with other devices. This gives clinicians more confidence that when the system does alarm, they can initiate timely intervention."
The significance of the COSMOS study
Patient mortality in the 30 days after surgery is 1,000 times higher than during the procedure.2 Many of these deaths stem from cardiovascular or respiratory complications. In the ward, where clinicians assess vital signs every four to eight hours, many of these life-threatening complications go unnoticed until it is too late, making continuous monitoring crucial to patient safety.
"Thirty-day postoperative mortality remains frighteningly common," says Daniel Sessler, MD, Michael Cudahy Professor and chair of the Department of Outcomes Research at Cleveland Clinic and founder of the Outcomes Research Consortium, anesthesia's largest academic research organization, in a Euroanesthesia 2020 webinar. "These deaths occur during the initial hospitalization—[with] patients under our care in the highest-level healthcare facilities."
In one study, only about 42% of patients had been checked by the nurse within two hours of a respiratory depression event.3 Monitoring of other values, such as oxygen saturation, heart rate, respiration rate, and activity, further helps clinicians rapidly detect anomalies, allowing them to begin potentially life-saving interventions.2
While an uninterrupted flow of data helps clinicians address post-surgical deterioration sooner, it also introduces complications. For one, traditional monitoring systems keep patients tethered to a bedside monitor, which may prolong recovery.4
Continuous monitoring devices also raise the potential for more false alarms. Alarm fatigue is a top contributor to clinician burnout and fatigue, and monitoring technology must alleviate this burden rather than exacerbate it.
How mobile continuous monitoring with actionable alarms helps improve patient care
Clinicians frequently turn to ECG-based telemetry monitoring to provide continuous mobile monitoring after surgery. Yet contrary to the proven benefits of telemetry in the management of cardiac diseases, an in-depth review of the medical literature doesn't yield a compelling level of evidence on its utility in non-cardiac conditions.5 Further, studies have shown that 80-99% of ECG monitor alarms are false or insignificant.6 The over reliance on ECG telemetry for non-cardiac patients may create a false sense of security and the high rate of false or insignificant alarms creates the potential for alarm fatigue.
In contrast to ECG monitoring, respiration rate (RR) has been shown to be a very important vital sign to measure in continuous monitoring since it is the best measurement for predicting clinical deterioration.7 RR increases when patients experience sepsis, acidosis, shock, pneumonia, and other conditions. Opioid-induced respiratory depression is one of the most dangerous consequences of opioid therapy after surgery. Without prompt treatment, serious respiratory depression can lead to brain injury and death.8
While RR is an important indicator of clinical deterioration, its standard monitoring methods may be either subjective or restrictive. For example, clinicians may monitor RR by watching a patient's chest rise and fall, an observation subject to variability. While traditional monitoring technologies like capnography provide objective readings, they require the patient to remain tethered to a medical device or to their bed.
Continuous mobile monitoring of RR also represents a more objective measure for clinicians, helping identify trends while also measuring RR spikes or dips instead of relying on periodic and potentially subjective human observation. Customization helps ensure alarms sound only in the case of sustained, actionable events and the visual trends may help clinicians identify potentially concerning changes even prior to alarming.
"If a patient's SpO2 or respiration rate drops, and it's not temporary, clinicians will want to know so they can take next steps," says Stahl. "By widening the measurement windows, adding alarm delays, and leveraging algorithms designed for mobile patients we reduce non-actionable alarms associated with transient events."
Facilitating enhanced recovery after surgery
Early mobility is a key component of the enhanced recovery after surgery (ERAS) pathways. Getting patients moving within the first 24 post-operative hours helps reduce risk of complications, accelerate recovery of walking capacity, and shorten length of stay.9
Traditional monitoring devices make it challenging for clinicians to comply with this element of the ERAS pathways. Meanwhile, a wireless solution helps prevent complications that result from immobility3 while enhancing the patient experience. Without wires getting in the way, a patient recovering from surgery can heal without worrying about disconnecting a device.
That said, mobile devices should be designed to have a stable signal to transmit patient data to the clinicians as well as an adequate algorithm for mobile patients, ensuring both sensitivity and specificity (and therefore increasing identification of true events while reducing the potential for false alarms).
Mobile monitoring: the next frontier in postoperative wards
Hospitals continuously monitor patients in operating rooms and intensive care units. However, after surgery, when the risks of complications and mortality are high, patients typically receive only occasional spot checks. A mobile monitoring solution that signals sustained, actionable events can help clinicians detect clinical deterioration before it becomes critical. With customizable thresholds, wards can tailor the device to patient needs.
The COSMOS study results make clear that customization improves usability for clinicians. Without the frustration of false alarms, clinicians can be confident in appropriate, 24/7 monitoring. Even better, without wires, patients can ambulate as needed for optimal recovery.
"We developed the technology with the goal of drastically reducing alarm burdens associated with continuous monitoring," says Stahl. "Yet we were pleasantly surprised by just how few alarms were shown by the Cleveland Clinic team after they analyzed and optimized the settings."
Learn more about GE HealthCare's Portrait Mobile solution.
Resources:
1. Anusic N et al. A1143. Alarm threshold and duration limits on medical-surgical wards through alarm burden modeling. Anesthesiology Annual Meeting. (2023)https://www.abstractsonline.com/pp8/ - !/10809/presentation/6664. Accessed November 2023.
2. Sessler D, & Saugel B. Beyond 'failure to rescue': the time has come for continuous ward monitoring. British Journal of Anaesthesia. (2019) 122(3):304-306. doi: 10.1016/j.bja.2018.12.003. Accessed November 2023.
3. Sun Z, Sessler, Daniel I., Dalton, Jarrod E., et al., Postoperative hypoxemia is common and persistent: a prospective blinded \observational study. Anesth Analg; 121: 709-15 (2015). https://doi.org/10.1213/ane.0000000000000836
4. Ljungqvist O et al. Enhanced Recovery After Surgery: A Review. JAMA Surgery. (2017) 1;152(3):292-298. https://pubmed.ncbi.nlm.nih.gov/28097305. Accessed November 2023.
5. Fayyaz B, Rehman HJ. 'Utilization of telemetry monitoring for non-cardiac conditions in non-critical patients: what are the trends and perceptions amongst medical residents?'. J Community Hosp Intern Med Perspect.;10(2):171-178. 2020. doi: 10.1080/20009666.2020.1759763. PMID: 32850059; PMCID: PMC7425609.
6. Jacques S, & Williams E. Reducing the safety hazards of monitor alert and alarm fatigue. Agency for Healthcare Research and Quality, PSNet. (2016) https://psnet.ahrq.gov/perspective/reducing-safety-hazards-monitor-alert-and-alarm-fatigue. Last Accessed November 2023.
7. Mochizuki K et al. Importance of respiratory rate for the prediction of clinical deterioration after emergency department discharge: a single‐center, case–control study. Acute Medicine & Surgery. (2): 172–178. (2017). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5667270. Accessed November 2023.
8. Respiration depression: respiratory depression can be an issue when intravenous agents are used. In Pharmacology and Therapeutics for Dentistry (Seventh Edition) (Eds. Dowd FJ et al.). (2017). https://www.sciencedirect.com/topics/medicine-and-dentistry/respiration-depression. Accessed November 2023.
9. Tazreean R et al. Early mobilization in enhanced recovery after surgery pathways: current evidence and recent advancements. Journal of Comparative Effectiveness Research. 11, 2. https://becarispublishing.com/doi/10.2217/cer-2021-0258. Accessed November 2023.








