Accurate monitoring of gas delivery and expiratory gases is essential to the care of ventilated patients. In this process, sampling lines play a crucial role in ensuring measurement accuracy, preventing nuisance monitoring alarms and maximizing patient safety.
First, it is important to understand the impacts anesthetic agents have on common sampling line materials and second, to appreciate how use of gas device manufacturer validated accessories can improve the function of anesthesia gas monitors.
Sampling lines and anesthetic agents
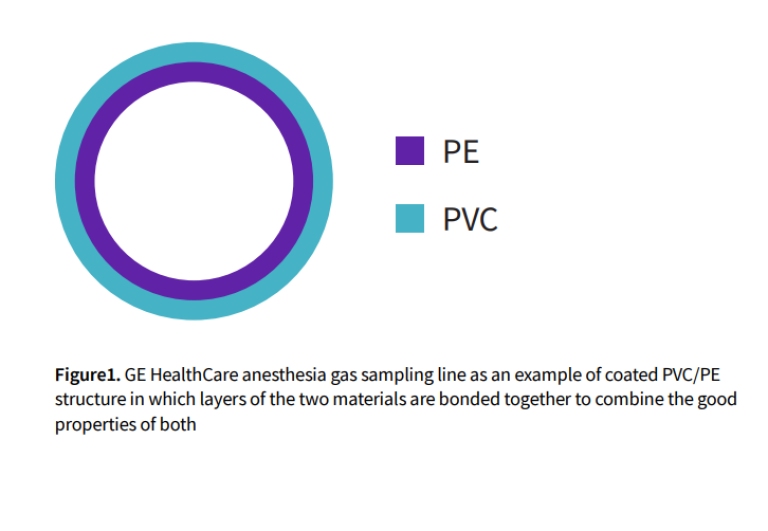
Sampling lines commonly marketed and used in anesthesia monitoring are made of Polyvinyl Chloride (PVC), Thermoplastic Elastomer (TPE) or bonded Polyvinyl Chloride/Polyethylene (PVC/ PE coextruded). GE HealthCare recommends the use of PVC/PE coextruded sampling lines in anesthesia applications.
Studies have been published1,2 on the compatibility of materials in the anesthesia circuit with anesthetic agents. Results of testing conducted by GE HealthCare with the most common sampling line materials have shown that a dedicated anesthesia gas sampling line is preferred for patient safety and to avoid nuisance monitoring alarms when anesthetic agents are present.
In recent decades, various tests confirm that common PVC tubing is an appropriate and cost-effective material for use in CO2 , O2 and N2 O sampling lines. However, if PVC tubing comes in direct contact with anesthetic agents, the accuracy of the measurement may be compromised. Therefore, PVC tubing with polyethylene (PE) interior coating is strongly recommended and preferred. The PE coating provides strong chemical resistance against volatile agents. Accuracy in measuring inspiratory and expiratory volatile anesthetic concentrations is especially important in low-flow anesthesia.
Understanding tubing plastics
Anesthetic agents are organic solvents and highly polar, surface active substances that tend to react with most commonly used plastics, including PVC. Pure PVC is hard and rigid. Plasticizers are often added during the manufacturing process to provide softness, flexibility and resistance to kinking and blockage with bending. In addition, PVC compounds may contain stabilizers to combat oxygen, sunlight and other environmental influences. However, the chemical properties of PVC tubing are still not optimal. The material may resist many chemicals but tends to lack durability against halogenated hydrocarbons (anesthetics) and aromatic hydrocarbons. Anesthetic agents and other organic liquids may dissolve the plasticizers, making the tubing hard and brittle. Conversely, PE has a stable structure and is inert against almost any chemical making it highly effective as a liner for anesthetic sampling tubing.
TPE as material in sampling lines makes it slightly more rigid than sampling lines made of PVC/PE.
Effects of anesthetic agents on PVC & TPE sampling lines
Since PVC compounds may contain plasticizers and other additives, two basic interactions may occur between PVC and anesthetics. If a PVC line is in contact with anesthetic, the anesthetic penetrates the plastic and swells it. At the same time, the plasticizer in the PVC dissolves into the anesthetic. In a test conducted by GE HealthCare, a PVC sampling line lost 5.4% of its weight in two hours due to liquid halothane dissolving. A PVC line coated in PE released no chemicals in an identical testing scenario.3
A similar interaction exists between gaseous anesthetics and PVC. In a test in which saturated anesthetic gases (desflurane and halothane) were conducted through sampling lines, oily PVC plasticizer residuals were observed on the tubing surfaces. Meanwhile, the sampling lines became brittle and stiff from loss of plasticizer. The same oily residuals were found on the filter membrane of the GE HealthCare D-Fend™ water trap, designed to dry and filter the sample of monitored gas.3
Oleoamides are used as fatty amide slip agents and lubricants in the manufacturing process of TPE sampling lines. Humidity and heat in the gas sampling line may cause leaching of these fatty substances and occlude the D-Fend water trap protective membrane, generating Replace Water Trap alarms and increasing the rise time of all anesthetic agents.4
Effects of sampling lines on gas measurement rise time
Tests3 demonstrate that interactions between anesthetics and PVC may influence monitoring response time. The PVC monomer contains one chlorine atom on its side. This halogen atom has many comprehensive effects on the material. When considering compatibility with anesthetic agents, one of the most important issues is the polarity that strongly electro-negative chlorine creates. While anesthetic agents contain many halogen groups, there is a strong chemical interaction between anesthetics and PVC.
Because of the polar interaction, PVC absorbs anesthetics onto the surface. It is therefore possible for a PVC sampling line to act as a temporary collector or supplier of the agent flow. This may cause underestimation of FiAA monitored values and overestimation of EtAA values. In testing, monitored rise times of gas measurements were virtually the same when CO2 , O2 and N2O were measured with PE-coated versus simple PVC lines. However, differences between the lines were considerable when anesthetics were measured. When PVC sampling line was used, the response time of halothane was five times longer than that of PE-coated sampling lines. Residuals anesthetic gases were observed in the monitored flow for as long as 15 seconds after feeding was stopped.
For the above discussed reasons, GE HealthCare recommends the use of PVC/PE Disposable anesthesia sampling lines in anesthesia applications.
Effects of sampling line inner diameter to response time
The GE HealthCare respiratory airway monitoring measurement is optimized for gas sampling lines of 1.2 mm (0.0472 in.) inner diameter. Gas sampling lines with a larger inner diameter will increase rise time, resulting in decreased performance with higher respiration rates. Gas sampling lines with a smaller inner diameter have higher flow resistance and are more prone to get blocked by water and secretions. This will cause additional Replace Water Trap or Sampling Line Blocked alarms on the monitor.
When monitoring Metabolics/Nutrition Gas Exchange, correct inner diameter and length of sampling lines (2m/7ft) are very important to ensure the measurement accuracies remain within specifications. Using sampling lines with a different inner diameter than 1.2 mm (0.0472 in.) may cause gas and flow signal synchronization issues in Gas Exchange measurement.
Why use device manufacturer validated gas sampling accessories
There is a vast quantity of accessory options available on the market. Device manufacturers design and optimize measurement algorithms together with specific accessories made available for use with their devices. Industry standards require all accessories used with a given patient monitor must be tested by the device manufacturer to validate monitoring performance, effectiveness and safety in use.
Conclusion
Selection of the appropriate sampling line material, inner diameter and correct deployment and handling of the sampling line and water traps can support measurement accuracy, prevention of nuisance alarms and maximized patient safety. Additional resources For white papers, guides and other instructive materials about GE HealthCare’s clinical measurements, technologies and applications, please visit http://clinicalview.gehealthcare.com/
References
- Eger El II. et al. Solubility of I-653, Sevoflurane, Isoflurane, and Halothane in Plastics and Rubber Composing a conventional Anesthetic Circuit. Anesth Analg 69, 218-25 (1989).
- Strum DP. et al. Stability of sevoflurane in soda lime. Anesthesiology 67, 779-81 (1987).
- Selecting adequate sampling line material, DOC2009086 4. VTT FTIR test report, DOC1985136
Not all products or features are available in all markets. Full product technical specification is available upon request. Contact a GE HealthCare Representative for more information.
Data subject to change.
© 2024 GE HealthCare
D-Fend is a trademark of GE HealthCare. GE is a trademark of General Electric Company used under trademark license.
All other trademarks are property of their respective owners.
Reproduction in any form is forbidden without prior written permission from GE HealthCare. Nothing in this material should be used to diagnose or treat any disease or condition. Readers must consult a healthcare professional.
JB50160XX_2 03/2024