Introduction
Innovation in healthcare takes many different forms, from novel implanted devices for brain or cardiovascular care, to automated intelligence solutions that better predict patients at risk for bacterial infections or other worrisome conditions. Other innovations may address clinician productivity, but they all share one common objective, to enhance clinical outcomes. One emerging innovation is in the use of hand gestures to silence bedside alarms, thereby freeing the clinician to dedicate more attention to the patient, while minimizing contact with hard surfaces that are linked with the spread of hospital acquired infections. What is noteworthy, is that not all innovation needs to be complex or leverage high tech concepts, rather a simple innovation like hand gesture alarm silencing provides an affordable and accessible tech solution that is clinician-centric and intuitive, and aims to relieve some of the burden faced with bedside alarms. This scoping review provides an outline of concepts that support the need for continued workforce simplification, combined with the ongoing need for improvements to infection control practices within the hospital.
Hospitals are facing worrisome demographic shifts
Prior to the onset of the global pandemic and since, hospitals globally were facing a convergence of two worrisome trends. On one hand, the complexity of patients being admitted has steadily increased for the past two decades. Central to that theme is the aging population, where in Europe, Eurostat projects that between 2019 and 2050, the very old population (age > 85 years) will double.1 The concern with an aging population, is that the number of comorbid conditions is positively correlated with age. Compounding this trend further is that there has been an increase in demand for emergency department services over the past decade. In the United States, there has been an 18.4% increase in emergency department utilization between 2006 and 2014, along with 6.9% increase in admissions.2 The combination of these two themes places increasing stress on the hospital staff to continually provide optimal care.
Consider the additional challenges within a hospital is the growing shortage of clinicians, both nurses and physicians. According to a survey by the American Hospital Association, nearly 50% of the hospitals surveyed reported critical care shortages prior to the pandemic.3 Since the COVID-19 pandemic, these labor shortages have only worsened.4 These labor shortages are nothing new, for the critical care societies as far back as 2004 called for Federal Action to avert the crisis in shortage for critical care physicians.5 The nurse to patient staffing ratios have deteriorated since the pandemic, where prior to the onset of COVID-19, the ratios were 2.0 to 1, while post-pandemic, the ratio worsened to 2.4 to 1.6 In short, critical care clinicians are tasked to doing more with less resources all the while taking care of increasingly more complex patients.
These changing demographics affecting patient complexity and clinician staffing will further challenge the hospital in providing high quality patient care, while minimizing distractions that include but are not limited to bedside alarms.
The burden of hospital acquired infections
Hospital acquired (or previously called nosocomial) infections have been a challenge facing hospitals for decades, with the US Centers for Disease Control and Prevention (CDC) establishing definitions in 1988.7 Simply stated, these are infections that develop during a hospital stay (including long-term care), but are not present nor incubating at the time of admission. Typically these infections develop within 48-72 hours following hospitalization and up to 10 days following hospital discharge. The incidence of hospital acquired infections (HAI) continues to rise, despite the fact that the average duration of hospital lengths of stay are decreasing.8,9 The effects of hospital acquired infections is broad, for these are unanticipated infections and can seriously impact both patient outcomes and hospital expenditures. Morbidity and mortality are known to increase in the face of HAIs, along with extending hospital lengths of stay and the need to undergo additional diagnostic tests and therapeutic interventions, all driving incremental costs to those already incurred.10
Bacteria and yeast frequently comprise the most common offending organisms and include examples such as methicillin resistant Staphylococcus aureus, vancomycin-resistant enterococci, Clostridium difficile and more esoteric yeasts such as Candida auris. 11 The most common mode of transmission of these offending organisms is through direct contact of an infected (or colonized) patient and a susceptible healthcare worker, from where the organism can spread to the next patient via touch, or adjacent hard surfaces.10
A variety of surfaces are known to become contaminated and serve as a reservoir for hospital-based pathogens to spread via cross-contamination, which include nursing uniforms, blood pressure cuffs, faucets and computer keyboards.12,13 In fact the hands are commonly implicated for cross-contamination as a result of touching non-patient surfaces. While clinicians are aware of the importance of hand hygiene practices following patient-contacting activities, what is often overlooked is hand washing following contact with hard surfaces. Pathogens associated with HAI have been shown to persist on hard surfaces for extended durations. In fact, common Gram positive organisms have been shown to persist for months on dry surfaces, while Gram negative organisms persist for longer durations when compared to Gram positive organisms.14 While yeast, such as Candida albicans can persist on hard surfaces for four months. There have been many efforts to control the spread of HAIs within the hospital and most facilities have implemented institutional environmental cleaning policies and procedures.15 The problem of cross-contamination remains, given the frequency of exposure to non-patient surfaces in a hospital.
Alarms and the cognitive impact on staff
A hospital is filled with medical devices that provide information to guide clinical decision making and include the ability to provide alerts and alarms. Alarms are essential to the delivery of optimal patient care, for they provide rapid notifications when changes in the patient’s clinical status arise. As advances in medical device technology have evolved, so too are the number and complexity of alarms that the clinician is exposed to. False alarms are those where no clinical or therapeutic action is needed. There are adverse consequences associated with false alarms, notably in the anesthesiology or critical care environments. In these environments, false alarms may not only be a disturbance, but can also be a risk factor to patients when critical alarms are ignored.16 The increased sound levels caused by alarms can have an adverse effect on clinicians and associations have been found between increased alarm sound levels and burn out in ICU nurses.17 In fact, one study of critical care nurses across Wisconsin found that noisy work environments were the greatest contributor to performance obstacles and may have impact on both nursing outcomes and patient safety.18
It is important to state that alarms are commonplace within the acute care setting and continual advancement in algorithm design has introduced “smart” alarms that can allow clinicians to customize triggers and notification parameters. The accuracy of alarm algorithms are constrained by multiple factors which include the complexity of clinical scenarios, variability in the disease states under care, sensor specifications and morphology of the human body (e.g. pigment, obesity, sweat, hair, etc.) The goal of alarm algorithm development is to provide the clinicians with increasingly more actionable information while minimizing the distraction that false alarms may provide. However, no alarm algorithm will be perfect given the limitations described above.
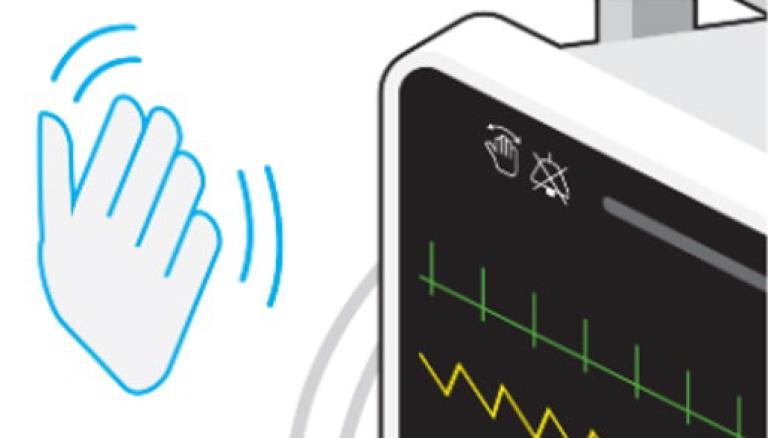
The advent of gesture alarm silencing Infection control in the hospital is a pervasive activity, for the health and economic impacts of an HAI are noteworthy. While infection control practices in a hospital have been long-established, certain frequently touched surfaces, such as patient monitors and the alarm silencing buttons, serve as a vulnerability to the control of contact transmission between patients. One recent innovation that may provide an incremental and intuitive mitigation is hand gesture alarm silencing. This feature can allow the bedside clinician, when an alarm sounds, to silence it via a simple hand wave gesture. In so doing, the clinician avoids contact with a frequently touched surface and thereby minimizing the risk of cross-contamination to other surfaces or patients. Gesture alarm silencing, in theory, may further improve clinician workflow, especially during bedside procedures. For example, when a bedside procedure (e.g. tracheal suctioning) is being performed and an alarm sounds, the nurse may be able to quickly assess the nature of the alarm and if appropriate, silence the alarm with a simple hand motion and thereby minimize distraction from the procedure at hand and reduce the stress associated with increased environmental sounds that are linked to increased clinician stress. Another scenario involves the nurse completing bedside charting, when an alarm sounds in an adjacent room. By use of a simple hand gesture, the alarm may be silenced quickly, while minimizing the risk for transmission of microbial pathogens between patients and allowing the nurse to return to completing their charting duties with less distraction.
While this innovation is only emerging in the clinical setting, hand gesture sensing technology has already been implemented outside of the hospital, such as calling for an elevator or scrolling channels on your smart television. The clinical utility for hand gesture silencing of alarms and the impact on hospital-acquired infections appears intuitive. However, there remains an opportunity to study the potential positive impact on patient safety and hospital economies and in turn play an incremental but potentially impactful role in relieving a small part of the burden caregivers face each day.
References
- European Commission. Statistical Office of the European Union. Ageing Europe: looking at the lives of older people in the EU : 2020 edition. [Internet]. LU: Publications Office; 2020 [cited 2023 Sep 16]. Available from: https://data.europa.eu/doi/10.2785/628105
- Lin MP, Baker O, Richardson LD, Schuur JD. Trends in Emergency Department Visits and Admission Rates Among US Acute Care Hospitals. JAMA Intern Med. 2018 Dec;178(12):1708– 10.
- Halpern NA, Pastores SM, Oropello JM, Kvetan V. Critical Care Medicine in the United States: Addressing the Intensivist Shortage and Image of the Specialty*. Critical Care Medicine. 2013 Dec;41(12):2754–61.
- Jatoi NN, Awan S, Abbasi M, Marufi MM, Ahmed M, Memon SF, et al. Intensivist and COVID-19 in the United States of America: a narrative review of clinical roles, current workforce, and future direction. Pan Afr Med J. 2022;41:210.
- Ewart GW, Marcus L, Gaba MM, Bradner RH, Medina JL, Chandler EB. The Critical Care Medicine Crisis: A Call for Federal Action: A White Paper From the Critical Care Professional Societies. Chest. 2004 Apr 1;125(4):1518–21.
- Greco M, De Corte T, Ercole A, Antonelli M, Azoulay E, Citerio G, et al. Clinical and organizational factors associated with mortality during the peak of first COVID-19 wave: the global UNITE-COVID study. Intensive Care Med. 2022 Jun 1;48(6):690– 705.
- Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. American Journal of Infection Control. 1988 Jun 1;16(3):128–40.
- Burke JP. Infection control - a problem for patient safety. N Engl J Med. 2003 Feb 13;348(7):651–6.
- Stone PW, Larson E, Kawar LN. A systematic audit of economic evidence linking nosocomial infections and infection control interventions: 1990-2000. Am J Infect Control. 2002 May;30(3):145–52.
- Collins A. Preventing Health Care–Associated Infections. In: Patient Safety and Quality: An Evidence-Based Handbook for Nurses [Internet]. Rockville, MD: Agency for Healthcare Research and Quality; 2008 [cited 2024 Aug 6]. Available from: https://europepmc.org/article/nbk/nbk2683#__NBK2683_dtls_ _
- Tsay S, Kallen A, Jackson BR, Chiller TM, Vallabhaneni S. Approach to the Investigation and Management of Patients With Candida auris, an Emerging Multidrug-Resistant Yeast. Clinical Infectious Diseases. 2018 Jan 6;66(2):306–11.
- Gastmeier P, Stamm-Balderjahn S, Hansen S, NitzschkeTiemann F, Zuschneid I, Groneberg K, et al. How Outbreaks Can Contribute to Prevention of Nosocomial Infection: Analysis of 1,022 Outbreaks. Infection Control & Hospital Epidemiology. 2005 Apr;26(4):357–61.
- Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental Contamination Due to Methicillin-Resistant Staphylococcus aureus: Possible Infection Control Implications. Infection Control & Hospital Epidemiology. 1997 Sep;18(9):622–7.
- Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006 Aug 16;6(1):130.
- HICPAC. Guidelines for Environmental Infection Control in HealthCare Facilities: Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) [Internet]. [cited 2024 Aug 6]. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5210a1.htm
- Schmid F, Goepfert MS, Reuter DA. Patient monitoring alarms in the ICU and in the operating room. Crit Care. 2013 Mar 19;17(2):216.
- Topf M, Dillon E. Noise-induced stress as a predictor of burnout in critical care nurses. Heart Lung. 1988 Sep 1;17(5):567–74.
- Gurses AP, Carayon P. Performance Obstacles of Intensive Care Nurses. Nursing Research. 2007 Jun;56(3):185.







