This white paper is also available in Spanish and in Portuguese.
Atelectasis during general anesthesia
Globally, more than 200 million patients undergo general anesthesia and receive mechanical ventilation every year.1-4 Overall, general anesthesia is an effective method for enabling surgical procedures, and mechanical ventilation is essential during general anesthesia. However, mechanical ventilation can also contribute to impaired oxygenation and gas exchange, primarily due to atelectasis – the partial or complete collapse of the whole lung or lobes.5, 6 Atelectasis is estimated to occur within minutes of anesthesia induction and can extend into the postoperative period for up to 90% of patients, making it one of the most common complications in the operating room.4-6 Compression of lung tissue, absorption of alveolar air and impairment of surfactant function are the three physiologic mechanisms5, 6 that may contribute to the development of atelectasis during general anesthesia.
- Compression atelectasis occurs when the diaphragm has displaced cephalad and relaxed, rendering it less effective in maintaining differential pressures between the thoracic and abdominal cavities. This causes a reduction in the transmural pressure, in turn distending the alveolus to a level that permits it to collapse.
- Absorption atelectasis occurs when a large volume of nitrogen in the lungs is replaced with oxygen. The oxygen may be subsequently absorbed into the blood, reducing the volume of the alveoli and resulting in alveolar collapse.
- Loss of surfactant atelectasis occurs when the pulmonary surfactant covering the large alveolar surface is compromised during exposure to anesthesia. This exposure may depress the stabilizing function of the surfactant and create alveolar collapse.
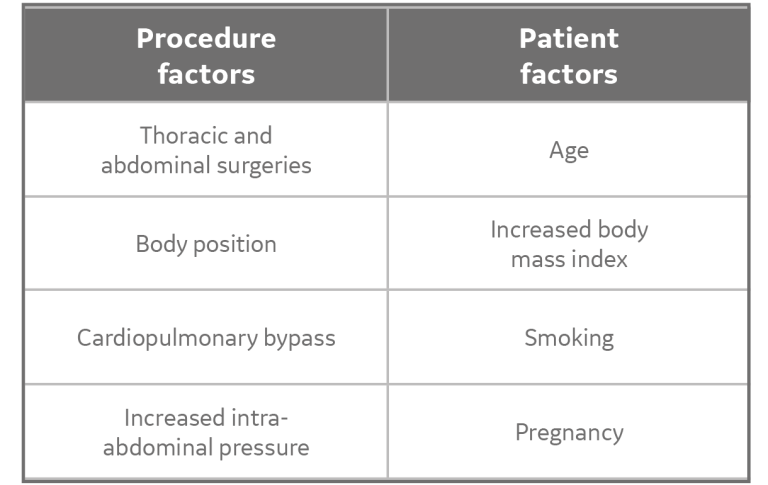
In addition to the three physiologic mechanisms, the following (not an exhaustive list) procedure and patient factors can influence the formation of atelectasis:
Atelectasis during general anesthesia contributes toward the development of postoperative pulmonary complications (PPCs). While the incidence rates of PPCs vary up to 23%,2 it has been demonstrated that compromised lung function increases:
(a) short- and long-term mortality
(b) morbidity
(c) health-care costs2
PPCs alone contributed to an 11.9% mortality rate in patients who underwent gastrointestinal procedures. In addition, an incremental postoperative ICU length of stay between 4.5 and 7.1 days corresponds with an associated increase in cost of $25,498.7
Intraoperative lung-protective ventilation:
summary of clinical evidence
While consensus exists that atelectasis during general anesthesia is a clinical problem with a health and financial impact, there is a multitude of opinions and schools of thought surrounding how to prevent lung collapse during general anesthesia.
Protective ventilation strategies have been used in critical care medicine and can be translated to the operating room with the aim of improving postoperative outcomes. Growing evidence suggests that prophylactic lung protective ventilation (LPV) strategies that use low tidal volumes (VT), moderate PEEP and recruitment maneuvers (RMs) may provide intraoperative protection by reducing the incidence of PPCs.1, 2, 4-6 This may lead to improved physiological and clinical postoperative outcomes. However, the role and ability of these strategies alone have yet to be fully understood. Reinius et al. demonstrated that in morbidly obese patients, PEEP and recruitment maneuvers, when applied individually, did not reduce atelectasis.8
However, a recruitment maneuver followed by PEEP did open atelectatic areas of the lung, improve arterial oxygenation, and increase respiratory system compliance in some cases.8
Lung recruitment maneuvers
Recruitment maneuvers (RMs) are intended to open collapsed alveoli by applying transient increases in transpulmonary pressure. In the following section, we will focus on single-step and multi-step recruitment as part of an intraoperative protective mechanical ventilation strategy.
Vital Capacity: single-step recruitment maneuver
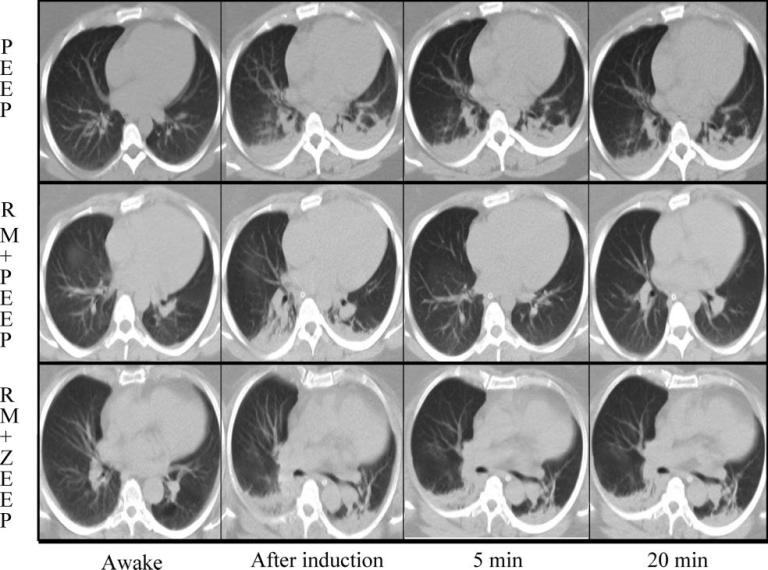
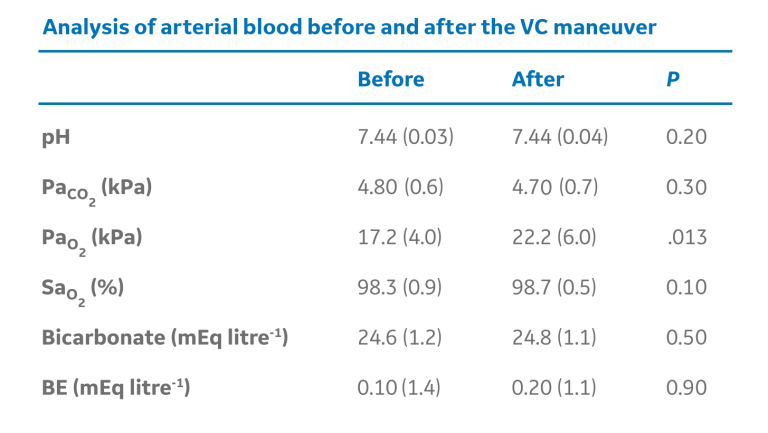
The manual “bag squeezing” or Vital Capacity (VC) method of applying and holding a set inflation pressure for a specific duration of time has proven to be effective in some cases. In patients undergoing neurosurgical or eye procedures, the inflation of healthy adult lungs to 40 cm H2O effectively re-expanded previously collapsed lung tissue within the first 7-8 seconds of the VC maneuver.9 The chart below demonstrates atelectasis along with the accompanying arterial blood gas measurements, before and after the “bag squeezing”, applied at least 15 minutes after anesthesia induction and held for 26 seconds.9
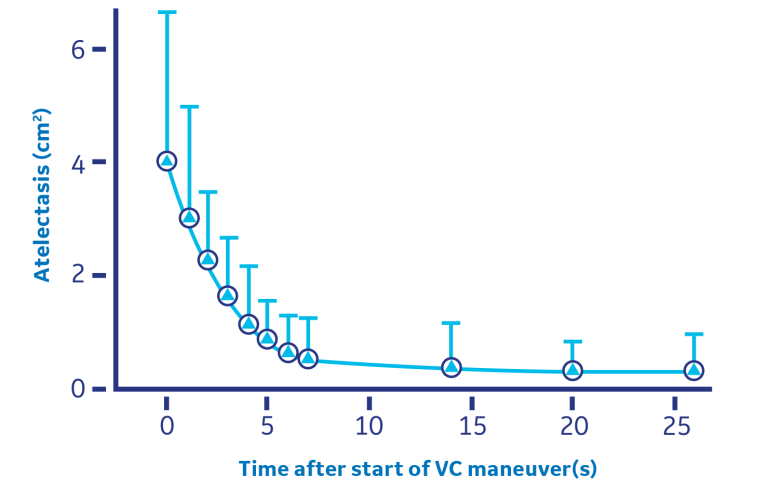
The manual “bag squeezing” or Vital Capacity (VC) method of applying and holding a set inflation pressure for a specific duration of time has proven to be effective in some cases.
While there is a no single approach that fits all patients, a recent meta-analysis offered the following observations:
- Reducing FiO2 in patient populations who can tolerate it can still provide adequate oxygenation while reducing atelectasis formation.
- A VC maneuver (+40 cm H2O for 15 seconds) coupled with PEEP (+10 cm H2O) is effective at preventing atelectasis formation and postoperative complications.
Other vital capacity recruitment strategies have also proven to be beneficial. Results from the Intraoperative Protective Ventilation (IMPROVE) study suggest that a prophylactic lung-protective ventilation strategy (in this case, VT of 6-8 ml/kg predicted body weight, PEEP of 6-8 cm H2O, and recruitment maneuver of 30 cm H2O for 30 seconds and repeated every 30 minutes), yields fewer postoperative complications in some cases.10
Cycling: multi-step recruitment maneuver
While single-step sustained-inflation strategies have been proven to be effective, multi-step (or stepwise) recruitment maneuvers may also be beneficial. Recruitment maneuvers employing an incremental increase in airway pressure and/or PEEP are known as stepwise RMs. These RMs allow for a more gradual increase in transpulmonary pressure. A stepwise RM strategy in pressure-controlled ventilation (ventilatory frequency of 15, I/E ratio of 1:1 and PEEP incrementally increased by 5 cm H2O from 0 cm H2O to 20 cm H2O) led to increased arterial oxygenation, expiratory lung volume, and respiratory compliance in patients undergoing open lower abdominal surgery.11 The alveolar recruitment strategy is summarized below. After the stepwise RM, as the data illustrates, dead space was reduced, CO2 elimination was improved, and ventilation efficiency in patients was improved.11
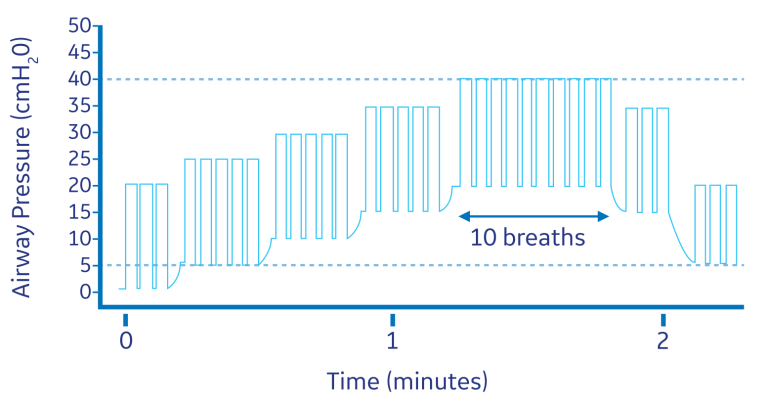
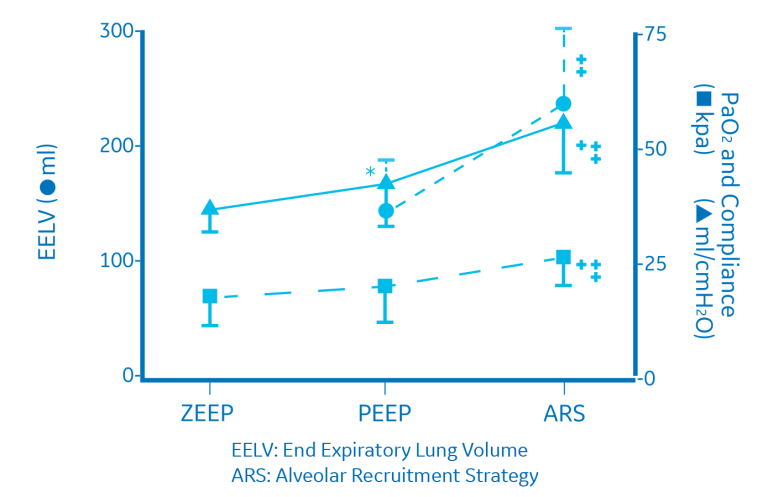
Lung protective technology solutions from GE Healthcare
Automated lung recruitment maneuvers: simplified workflow to help clinicans with patient care
GE Healthcare’s latest anesthesia delivery systems are designed to simplify workflow and help clinicians provide more effective patient care. In the latest software releases for the GE Anesthesia Aisys CS2, Avance CS2 and Carestation 600 Series, lung recruitment maneuver procedures have been automated. They are referred to as Vital Capacity and Cycling under the Procedures key. These automated capabilities allow the clinician to perform the procedures with accuracy and efficiency.
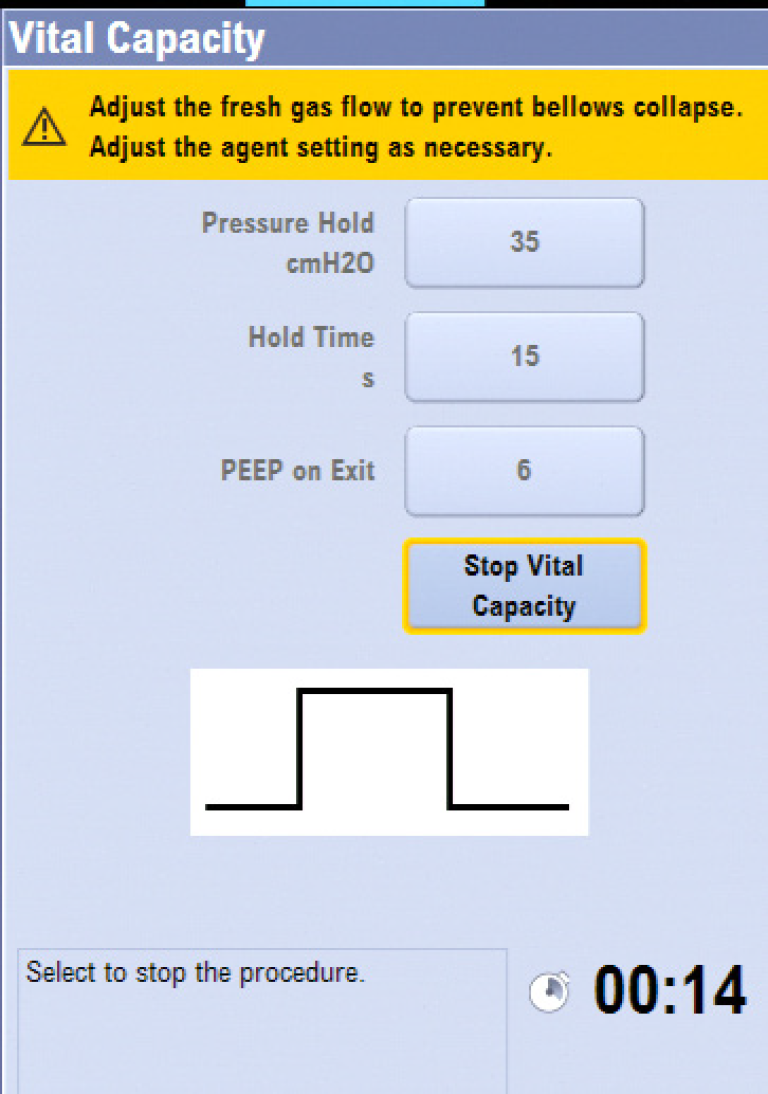
Vital Capacity
The Vital Capacity feature automates the manual bag “squeeze and hold” and provides a simple way to deliver one pressure breath without making multiple ventilator setting changes. The PEEP on Exit setting provides a way to change the ventilator PEEP setting automatically at the end of the Vital Capacity procedure.
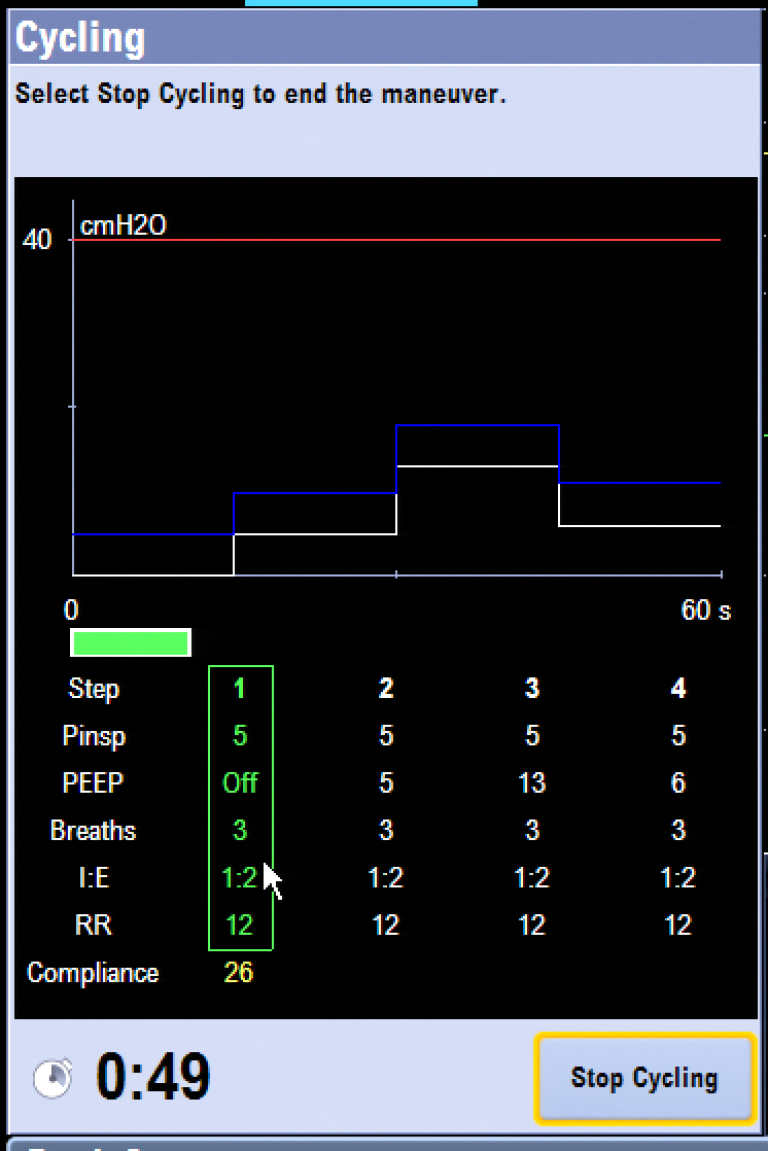
Cycling
The Cycling feature automates the multi-step recruitment maneuver. It is an automated, programmable feature that allows clinicians to increase and decrease PEEP levels during mechanical ventilation. The Cycling feature provides a flexible way to deliver pressure breaths during ventilation without making multiple ventilator setting changes. Four cycling profiles with up to seven preset steps are available. Each procedure’s default steps and ventilation settings can be preset by the Super User. The ventilation settings of each step can be changed by the user before starting a procedure. In the latest version of the Aisys CS2 software, the active step can be visualized in a green box along with the trended compliance measurements that show the real time effectiveness of automated lung procedures.
Patient spirometry: lung compliance as a fundamental parameter in assessing the efficacy of recruitment during anesthesia
Lung compliance reflects the distensibility of the respiratory system. It is defined as a pressure difference required to expand the lung by a certain volume. Dynamic pulmonary compliance is calculated by dividing VT by the difference between PIP and PEEP: dynamic compliance = VT/(PIP - PEEP). As a continuous dynamic value, it provides an easy tool for the clinician to follow respiratory changes and to adjust ventilator settings accordingly.
An increase in pulmonary compliance immediately following the application of an alveolar recruitment maneuver would presumably reflect a reduction in atelectasis and result in an increase in PaO2 due to better matching of ventilation and perfusion.
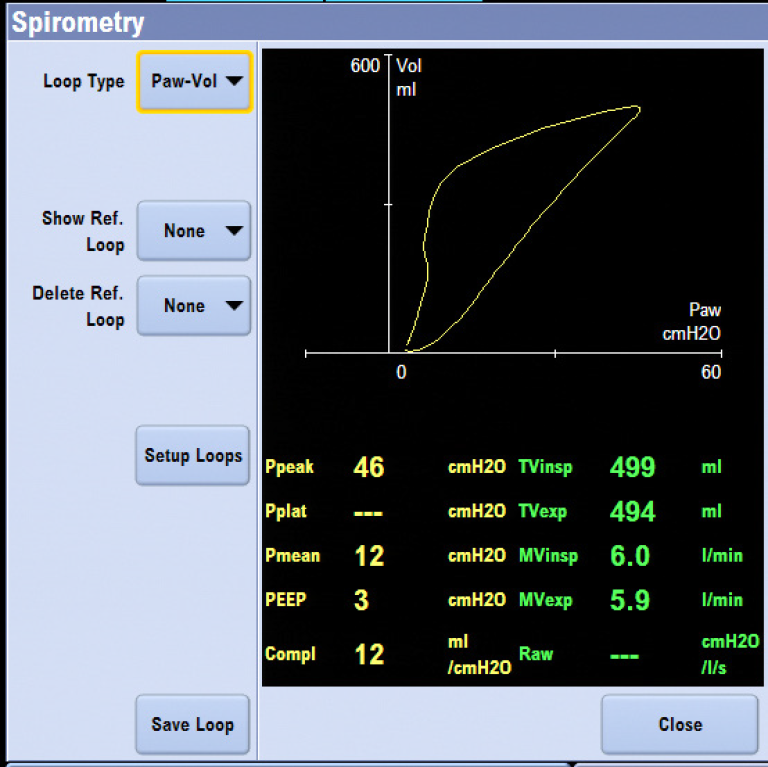
Patient Spirometry, featured on GE Anesthesia Aisys CS2, Avance CS2 and Carestation 600 Series, measures airway pressures, flow, volumes, compliance and airway resistance, breath-by-breath, at the patient’s airway. The dynamic interrelationships of pressure and volume, flow and volume, and pressure and flow are displayed as graphical loops.
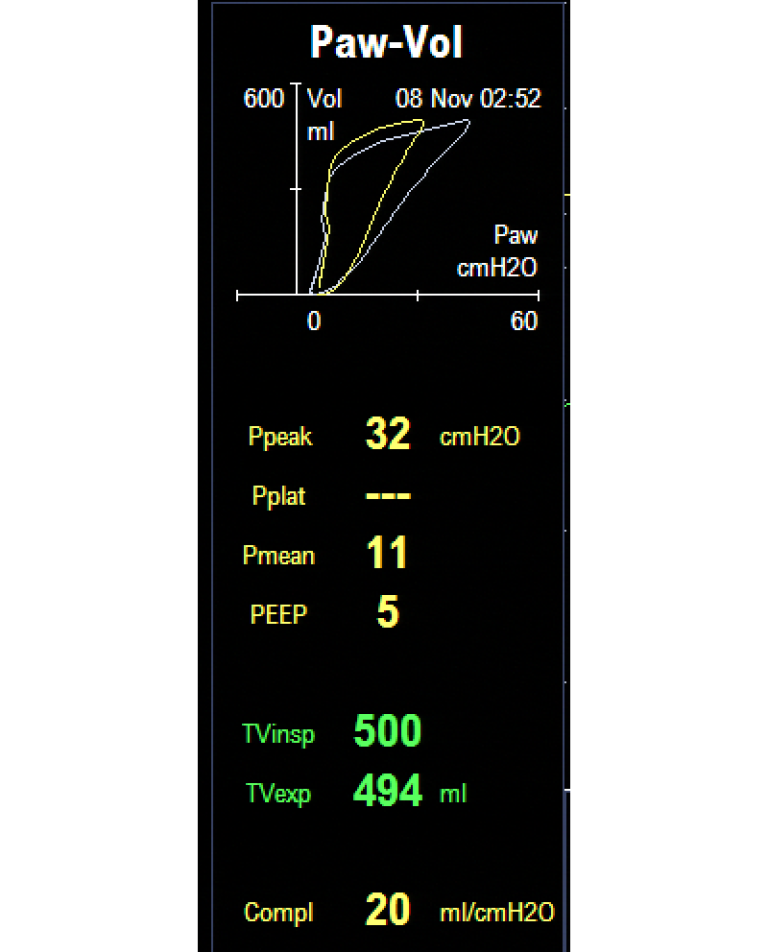
This figure demonstrates effects of different PEEP settings (and hence recruitment maneuvers) on patient compliance. The saved loop (white) illustrates decreased compliance. The situation is altered by increasing the PEEP setting to 5 cmH2O which clearly improves the lung compliance (yellow).
During a cycling procedure, the dynamic compliance value will be displayed at each PEEP level, thus allowing the clinician to understand when recruitment is achieved and the optimal PEEP level for that specific patient.
Pulse pressure variation (dPP) and systolic pressure variation (SPV): continued monitoring of patients’ hemodynamic stability
Positive pressure ventilation causes blood pressure changes in the chest cavity. Blood pressure increases during the inspiratory phase and decreases during the expiratory phase. The magnitude of these changes is dependent on the fluid status of the patient. In hypovolemic patients, these swings are higher in amplitude compared to normovolemic or hypervolemic patients. This is a well-known phenomenon.
The Pulse Pressure Variation (dPP) and Systolic Pressure Variation (SPV) tools found on GE Healthcare’s CARESCAPE Patient Monitors (B850, B650 and B450) along with the Aisys CS2, Avance CS2 and Carestation 600 Series can be used to effectively perform an individualized, patient-centric lung protective strategy. dPP and SPV are clinical decision tools that may help predict hemodynamic instability induced by positive end-expiratory pressure and the application of recruitment maneuvers.
A rise in intrathoracic pressure might increase the right ventricular afterload, compress the intrathoracic veins, and reduce the cardiac output. The lungs may also exert a compressive effect on the heart and impair cardiac compliance. The variation of cardiac load may be reflected in dPP and SPV, therefore the clinician can leverage the dPP and SPV value to assist with the decision regarding the potential impact of lung recruitment on hemodynamics.
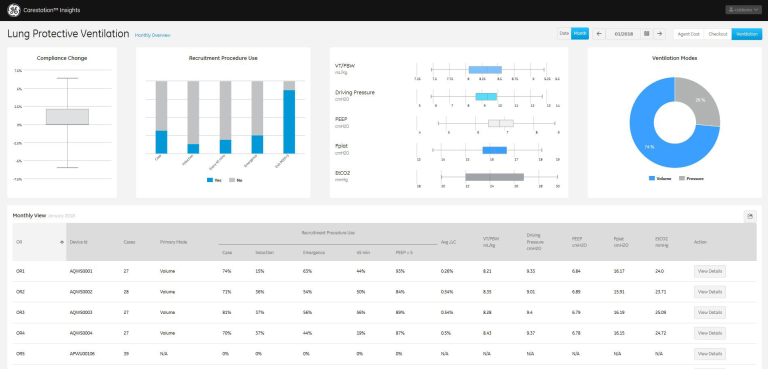
Carestation Insights: anesthesia data analytics to provide increased visibility into ventilation and lung response
Carestation Insights is a cloud-based suite of analytics applications designed to help clinicians make data driven decisions to improve outcomes. It analyzes over 300 data points covering ventilation values, gas values, alarms, error codes and machine status.
The Lung Protective Ventilation application tracks ventilation settings and responses across all connected anesthesia machines. It provides data for hospitals to support their lung protection initiatives to drive improved clinical outcomes and help reduce post-operative complications.
This application provides the visibility necessary to drive behavioral change. It provides a tracking mechanism for continuous data and trends of key ventilation settings and parameters, allowing both a department level and OR-level view of LPV protocol compliance. Additionally, a cause-and-effect relationship between ventilation settings and intraoperative patient outcome through a greater awareness of changes in patient lung compliance can help with the education of clinicians on the impact of their practice on their patients’ outcomes.
GE Healthcare provides a holistic solution to monitor, deliver and activate each aspect of a lung protective ventilation strategy. To determine which GE solution is best for you, contact your GE Healthcare representative or visit www.gehealthcare.com
References
- E. Futier, E. Marret, S. Jaber, Perioperative positive pressure ventilation: an integrated approach to improve pulmonary care. Anesthesiology 121, 400-408 (2014).
- A. Miskovic, A. B. Lumb, Postoperative pulmonary complications. Br J Anaesth 118, 317-334 (2017).
- N. M. Goldenberg, B. E. Steinberg, W. L. Lee, D. N. Wijeysundera, B. P. Kavanagh, Lung-protective ventilation in the operating room: time to implement? Anesthesiology 121, 184-188 (2014).
- A. Güldner et al., Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications: a comprehensive review of the role of tidal volume, positive end-expiratory pressure, and lung recruitment maneuvers. Anesthesiology 123, 692-713 (2015).
- M. Duggan, B. P. Kavanagh, Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiology 102, 838-854 (2005).
- L. Magnusson, D. R. Spahn, New concepts of atelectasis during general anaesthesia. Br J Anaesth 91, 61-72 (2003).
- L. A. Fleisher, W. T. Linde-Zwirble, Incidence, outcome, and attributable resource use associated with pulmonary and cardiac complications after major small and large bowel procedures. Perioper Med (Lond) 3, 7 (2014).
- H. Reinius et al., Prevention of atelectasis in morbidly obese patients during general anesthesia and paralysis: a computerized tomography study. Anesthesiology 111, 979-987 (2009).
- H. U. Rothen et al., Dynamics of re-expansion of atelectasis during general anaesthesia. Br J Anaesth 82, 551-556 (1999).
- E. Futier et al., A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 369, 428-437 (2013).
- G. Tusman, S. H. Böhm, F. Suarez-Sipmann, E. Turchetto, Alveolar recruitment improves ventilatory efficiency of the lungs during anesthesia. Can J Anaesth 51, 723- 727 (2004).