An accuracy study of TruSignal SpO₂ technology during challenging patient conditions
Mikko Hyle, M.Sc.
Introduction
Pulse oximetry, or SpO₂, is a clinical measurement typically used throughout many different care areas and applications. Assessing a patient’s oxygenation level is one of the key elements in helping clinicians develop the best course of treatment for their patients. However, sometimes there are clinical motion artifacts or low-perfusion conditions that can affect the accuracy of pulse oximetry monitoring. In order for a clinician to determine an optimal treatment plan, it is crucial that the clinicians’ SpO₂ technology is reliable and accurate under these circumstances.
Summary
The accuracy of GE Healthcare’s SpO₂ technology, TruSignal™, was tested in three studies. Two studies were conducted in challenging clinical conditions and one study in normal conditions. The challenging conditions included low-perfusion, motion and non-motion situations.
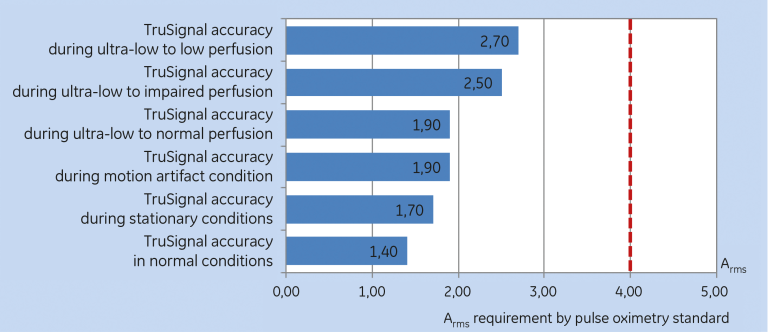
The results showed that the SpO₂ accuracy (Arms)1 of the TruSignal technology was under two digits in the normal, motion and non-motion conditions. The accuracy result for the low-perfusion condition showed under three digits. All results exceed the requirement of the ISO 9919 standard of four digits. see Figure 1.
Study method
The SpO₂ accuracy evaluation during normal conditions was conducted in accordance with ISO 9919, Arterial Blood CO-Oximetry during Induced Hypoxia tests over the range of 70-100 percent SaO₂. Eleven normal, healthy subjects with skin tones ranging from light to dark were tested for evaluation. GE Healthcare’s CARESCAPE™ V100 vital signs monitor with an Ohmeda™ TruSignal OEM board was used. see Figure 2.
Ten normal subjects with skin tones ranging from light to dark were tested for accuracy during motion and non-motion conditions. For the motion test, one of the three defined motions was used: mechanically induced tapping, clinical rubbing motion, or randomized clinical motion. The CARESCAPE V100 vital signs monitor with an Ohmeda TruSignal OEM board was used. See Figure 2.
Eight normal subjects with skin tones ranging from light to dark were tested for accuracy during a low-perfusion condition. In the low-perfusion test the subject was moved to a cooled room, and the subject’s side with the arterial line in place was kept warm. The subject’s other side was cooled to a low-perfusion level. Perfusion was measured in relative perfusion index.² The monitor used was the GE Datex-Ohmeda TruSat™ pulse oximeter with TruSignal technology. see Figure 3. GE Healthcare’s OxyTip+™ sensors were used in all studies.³

In the two challenging conditions’ studies the SpO₂ values were collected simultaneously by blood being drawn from the indwelling catheter in the left radial artery of each subject. The blood was immediately analyzed by CO-Oximeters to determine arterial oxygen saturation, SaO₂. The accuracy of the TruSignal algorithm is stated in Arms, which is the root mean square difference between the measured SpO₂ values and the CO-Oximetry (SaO₂) reference values. It is important to note that the TruSignal technology was calibrated against CO-Oximeters, as it is considered the most accurate method to define hemoglobin oxygenation due to its invasive nature.
STUDY RESULTS
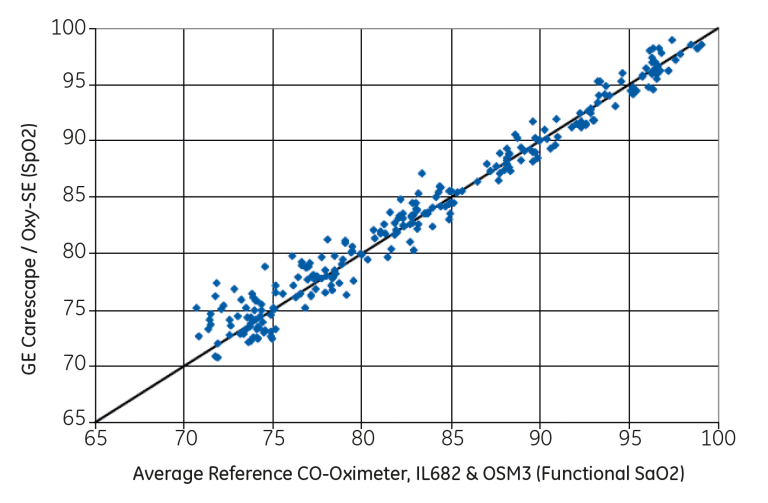
In the normal condition study, using the OxyTip Sensitive Skin sensor, 261 data points were collected in the oxygen saturation range of 70-100 percent with an accuracy of Arms of 1.4. ISO standards require a score lower than 4.0.⁴ see Figure 4.
In the study for accuracy during motion and non-motion conditions, SpO₂ values in the oxygen saturation range of 70-100 percent was measured 324 times in stationary conditions and 543 times in motion artifact conditions. Accuracies of the TruSignal technology were 1.7 and 1.9, respectively.
In the study for accuracy during low perfusion conditions, SpO₂ values in the oxygen saturation range of 70-100 percent was measured for three perfusion levels: ultra-low to normal, ultra-low to impaired and ultra-low to low. The accuracy of the TruSignal technology was 1.9 in ultra-low to normal perfusion, 2.5 in ultra-low to impaired perfusion and 2.7 in ultra-low to low perfusion. see Table 5.

Conclusion
In a study consisting of both normal and challenging patient conditions, GE Healthcare’s TruSignal SpO₂ technology exceeded the accuracy requirements set by the ISO 9919.
References
1. Arms: Accuracy root mean square,
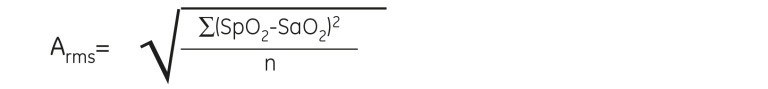
Two thirds of the data points are expected to fall within the Arms requirement.
2. Relative perfusion index can be changed to modulation % bythe formula PIr*3 = IR Mod %
3. Since this study was conducted, GE Healthcare developed a TruSignal portfolio of sensors, which performs better than the OxyTip+ sensors, and includes an additional new FingerTip sensor style. “Overview of TruSignal SpO₂ cable and connector performance” DOC0724831.
4. The SpO₂ accuracy requirement of pulse oximeter equipment by “BS EN ISO 80601-2-61:2011 Medical electrical equipment. Particular requirements for basic safety and essential performance of pulse oximeter equipment” –standard.
Study documentation
This paper is based on the following internal GE documentation:
Final Test Report For GE Healthcare Results of the SpO₂ Accuracy Performance of the GE Healthcare CARESCAPE, ProCare with Ohmeda TruSignal OEM board with GE Healthcare OxyTip+ Sensors as Compared to Simultaneous Arterial Blood Functional Oxygen Saturation Values During Non-Motion Conditions of Stable Hypoxia.*
Results of SpO₂ Accuracy of Datex-Ohmeda TruSat Pulse Oximeter (ver. .43 / .42) with OxyTip+ Sensors During Low Perfusion Conditions as Compared to CO-Oximetry
Results of Oxygen Saturation Testing of TruSat Pulse Oximeter (ver .42 / .43) During Motion and Non-Motion Conditions Compared to Arterial Blood CO-Oximetry
* The monitor is commercially available as the CARESCAPE V100 monitor.
Additional resources
For white papers, guides and other instructive materials about GE Healthcare’s clinical measurements, technologies and applications, please visit http://clinicalview.gehealthcare.com/







