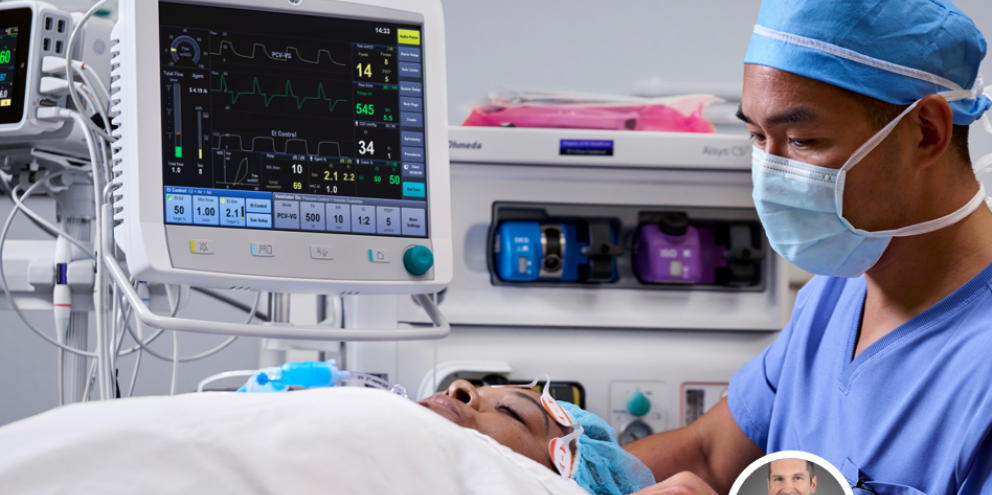
In April 2022, GE HealthCare earned FDA approval as the first software that helps automate anesthesia delivery in the U.S by conducting a MASTER-Anesthesia Trial (Multi-site Anesthesia randomized controlled STudy of End-tidal control compared to conventional anesthesia Results).
Kyle Herzog, an engineer with GE since 2008, served as the lead engineer on that clinical trial of GE’s End-tidal control* software, which involved 248 patients at four institutions.
In this second video of our three-part series, Herzog delivers a technical overview, examining:
- End-tidal control software and hardware components
- How End-tidal control works
- Which patients benefit from End-tidal control
- Safety mechanisms and checks
- How GE gained FDA-approval
- Results and observations from that US clinical trial
*Et Control is indicated for patients 18 years of age and older in the United States.
©2024 GE HealthCare
GE is a trademark of General Electric Company used under trademark license. Reproduction in any form is forbidden without prior written permission from GE HealthCare. Nothing in this material should be used to diagnose or treat any disease or condition. Readers must consult a healthcare professional.
JB29328XX, JB29289XX

Kyle Herzog
Engineering Manager, GE HealthCare – Anesthesia & Respiratory Care
Kyle Herzog has worked as an Engineer at GE HealthCare in Madison, WI since 2008 after graduating with a Biomedical Engineering degree from UW-Madison. He has worked on the Verification and Validation (V&V) team for his entire career with a focus on creating high quality and reliable Anesthesia Machines. He has worked with End-Tidal Control the majority of his career and was a lead engineer on the MASTER trial that eventual lead to FDA Approval of the Et Control feature.