How is Respiration Rate Monitored?
GE HealthCare bedside patient monitors provide capability to monitor respiration rate during ECG monitoring. Impedance respiration is measured across the thorax and upper abdomen between ECG electrodes. The respiration signal is made by supplying current between the electrodes and by measuring the differential current from the electrodes. The signal measured is the impedance change caused by breathing
Lead Placement options
There are three lead placement options available in GE HealthCare bedside patient monitors. These are Lead I, Lead II and Vector RL-LL .
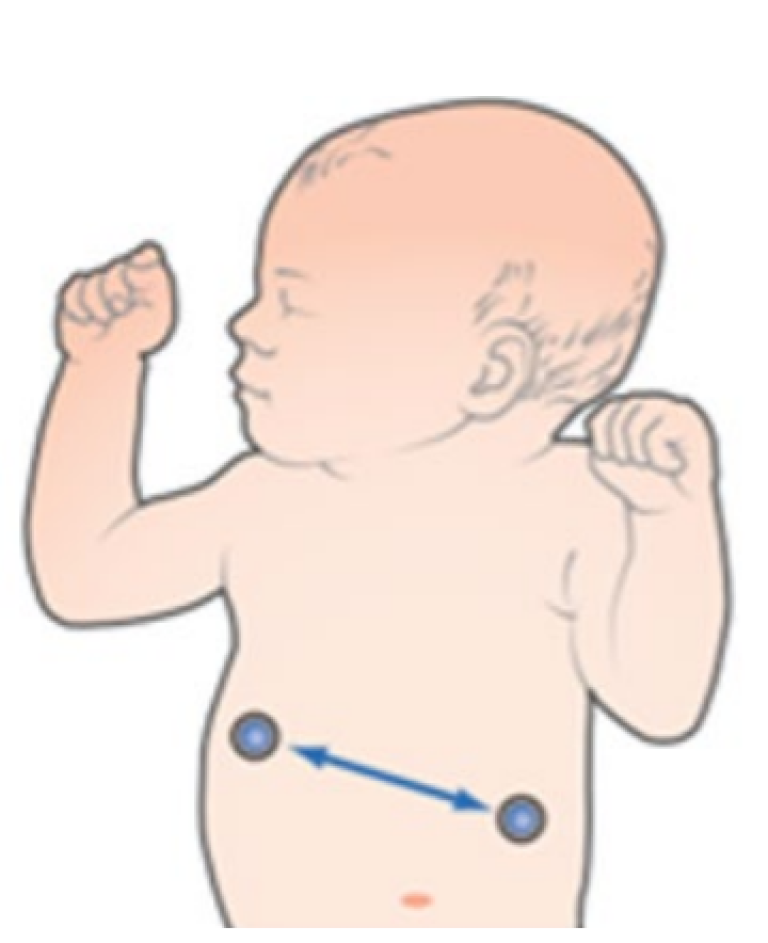
Lead I
Provides good thoracic (upper chest) breath detection. However, lead I is more susceptible to cardiogenic artifact than the RL-LL vector.
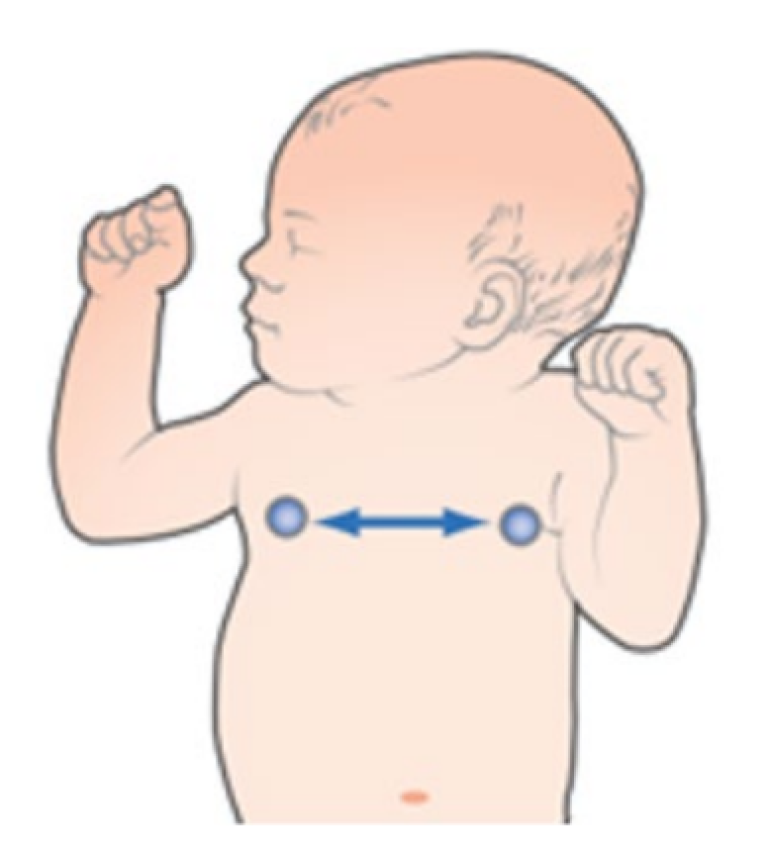
Lead II
Provides good thoracic breath detection and upper abdominal (lower chest) breath detection. However, lead II is more susceptible to both cardiogenic and motion (head, neck, or arm) artifact than the RL-LL vector.
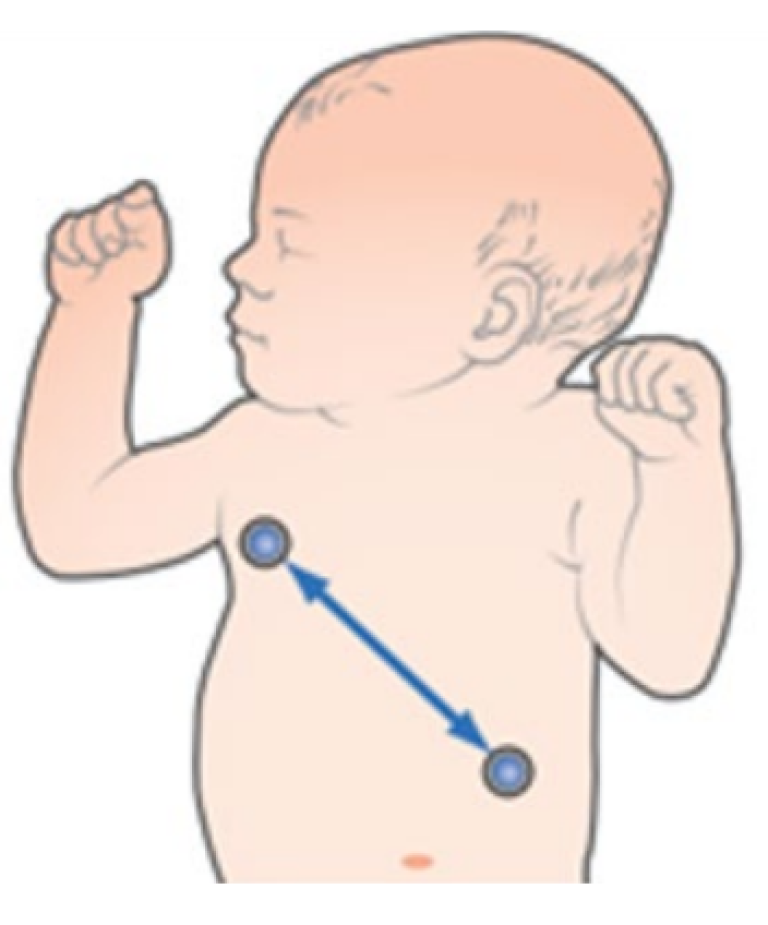
Vector RL-LL
Provides good abdominal breath detection and is not as susceptible to cardiogenic artifact or motion artifact. However, RL-LL is not available with 3-lead placement and with 5-,6- and 10-leadwire electrode placement, the RL electrode should be placed on the fifth intercoastal space on the right side of the chest.
Optimal Sensitivity
Choosing a vector that expands over the abdomen is beneficial for achieving good signal amplitude and high signal to noise ratio with neonates.1 Hence selecting vector RL-LL is preferred for neonates for reaching the highest sensitivity for the respiration rate monitoring.
Additional resources
For white papers, guides and other instructive materials about our clinical measurements, technologies and applications, please visit http://clinicalview.gehealthcare.com/
References:
- Optimal Electrode Location for Monitoring the ECG and Breathing in Neonates. R. Neuman et all. Pediatric Pulmonology 12:247-250 (1992)
- Clinical Reference Guide - CARESCAPE B850/B650/B450 Modular Monitors, CARESCAPE ONE (DOC2629462)
- User’s Manual - Patient Monitor B155M/B125M/B125P/B105M/ B105P V3 (5828000-EN Rev. 4)
Not all products or features are available in all markets. Full product technical specification is available upon request. Contact a GE HealthCare Representative for more information. Data subject to change.
© 2024 GE HealthCare
GE is a trademark of General Electric Company used under trademark license. All other third-party trademarks are the property of their respective owners.
Reproduction in any form is forbidden without prior written permission from GE HealthCare. Nothing in this material should be used to diagnose or treat any disease or condition. Readers must consult a healthcare professional.
JB28596XX 03/2024









