Background
- Pulse oximetry (SpO2) is the standard of care for assessing oxygen saturation in the acute care setting1
- Low-perfusion degrades pulse oximetry performance and represents a clinical challenge2
- Manufacturers have developed pulse oximetry technologies to minimize the impact of artifacts on sensor performance
Objectives
- A comparative study was conducted to evaluate SpO2 accuracy during low-perfusion conditions among three currently available devices: GE HealthCare CARESCAPE, Masimo RADICAL-7 and Medtronic Nellcor PM1000N
Methods
- After University of California San Francisco IRB approval, healthy adult ( ≥18 years) volunteer non-smokers with normal Hgb levels were recruited for this prospective, open-labeled study
- Testing was conducted using a minimum of 10 subjects, including ≥2 subjects with darkened skin pigment (FDA Pulse Oximeter Guidance (2013) & ISO 80601-2-61:2017)
- Skin pigmentation was categorized by the Fitzpatrick scale
- Low perfusion was simulated using ice-bath immersion with the left arm, while the right arm served as the control
- All 3 pulse oximeters were placed on both hands, using a randomized, counter-balanced approach for SpO2 finger placement to control for order bias
- SpO2 readings were compared between the normal perfusion and low perfusion hands to measure accuracy during low perfusion
- The Perfusion Index (PI), calculated as the ratio of pulsatile component to non-pulsatile component of the infrared plethysmographic signal, served as the measurement standard for flow: <0.1=ultra-low; ≥0.1 to <0.3=very low, ≥0.3 to <1=low; and ≥=normal
- Induced hypoxia was used to provide a range of oxygen saturation levels (normal:>90% and low: 75-85%) by having subjects breathe mixtures of nitrogen, room air, and carbon dioxide
- Descriptive data for comparison included the Accuracy Root Mean Square (ARMS), bias, and absolute delta (AD)
Results
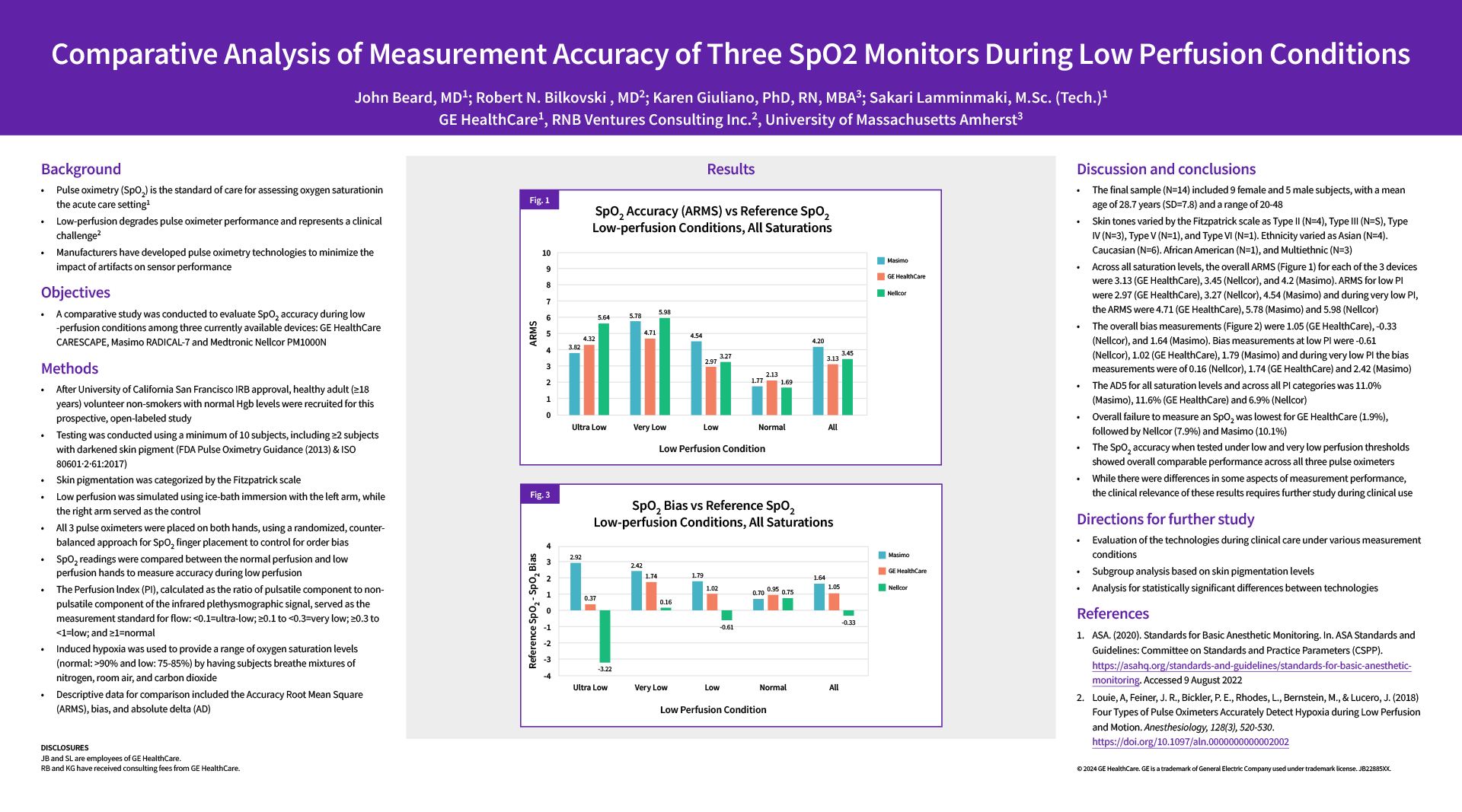
- Fig. 1: SpO2 Accuracy (ARMS) vs Reference SpO2. Low-perfusion conditions, all saturations
- Fig. 2: SpO2 Bias vs Reference SpO2. Low-perfusion conditions, all saturations
Discussion and Conclusions
- The final sample (N=14) included 9 female and 5 male subjects, with a mean age of 28.7 years (SD=7.8) and a range of 20-48
- Skin tones varied by the Fitzpatrick scale as Type II (N=4), Type III(N=5), Type IV (N=3), Type V(N=1), and Type VI(N=1). Ethnicity varied as Asian (N=4), Caucasian (N=6), African American (N=1), and Multiethnic (N=3)
- Across all saturation levels, the overall ARMS (Figure 1) for each of the 3 devices were 3.13 (GE HealthCare), 3.45 (Nellcor), and 4.2 (Masimo). ARMS for low PI were 2.97 (GE HealthCare), 3.27 (Nellcor), 4.54 (Masimo) and during very low PI, the AEMS were 4.71 (GE HealthCare), 5.78 (Masimo) and 5.98 (Nellcor)
- The overall bias measurmenets (Figure 2) were 1.05 (GE HealthCare), -0.33 (Nellcor), and 1.64 (Masimo). Bias measurements at Low PI were -0.61 (Nellcor), 1.02( GE HealthCare), 1.79 (Masimo) and during very low PI the bias measurment were of 0.16 (Nellcor), 1.74 (GE HealthCare) and 2.42 (Masimo)
- The AD5 for all saturation levels and across all PI categories was 11.0% (Masimo), 11.6%( GE HealthCare) and 6.9% (Nellcor)
- Overall failure to measure and SpO2 was lowest for GE HealthCare (1.9%), followed by Nellcor (7.9%) and Masimo (10.1%)
- The SpO2 accuracy when tested under low and very low perfusion thresholds showed overall comparable performance across all three pulse oximeters
- While there were differences in some aspects of measurements performance, the clinical relevance of these results requires further study during clinical use
Directions for further study
- Evaluation of the technologies during clinical care under various measurements conditions
- Subgroup analysis based on skin pigmentation levels
- Analysis for statistically significant difference between technologies
References
- ASA. (2020). Standards for Basic Anesthetic Monitoring, In. ASA Standards and Guidelines: Committee on Standards and Practice Parameters (CSPP). https://www.asahq.org/standards-and-guidelines/standards-for-basic-anesthetic-monitoring. Accessed 9 August, 2022
- Louie, A, Feiner, J-R-, Bickler, P.E., Rhodes, L., Bernsteing, M., & Lucero, J. (2018). Four Types of Pulse Oximeters Accurately Detect Hypoxia during Low Perfusion and Motion. Anesthesiology, 128(3), 520-530. https://doi.org/10.1097/aln.0000000000002002
Disclosures:
JB and SL are employees of GE HealthCare
RB and KG have received consulting fees from GE HealthCare