Introduction
Inhaled anesthetic delivery requires precise control to ensure patient safety. Traditional manual adjustments of fresh gas flows and anesthetic vaporizer settings require repetitive user actions to maintain anesthetic and oxygen concentrations at desired levels. In practice, the anesthesia provider adjusts the fresh gas flow and vaporizer settings in real-time in response to changes in patient condition and surgical events, while simultaneous additional actions of patient care may be required in the dynamic surgical environment. Moreover, the cognitive burden associated with manual tasks may contribute to human error during the care of surgical patients1. End-Tidal Control* (EtC) automates many of the required manual gas flow and anesthetic adjustments and has been utilized globally for over a decade. Its introduction in the United States offers a promising solution to reduce repetitive manual tasks while improving the accuracy and efficiency of anesthesia delivery.
A randomized controlled study known as the MASTER Trial (Multi-site Anesthesia Randomized Control STudy of End Tidal Control Compared to Conventional Anesthesia Results) was conducted across four U.S.-based hospitals prior to FDA approval of EtC. The MASTER Trial assessed the safety and efficacy of EtC compared to conventional manual control (MC) in achieving and maintaining targeted end-tidal anesthetic agent (EtAA) and end-tidal oxygen (EtO2) concentrations during inhalational anesthesia delivery. The findings of the MASTER Trial have been peer-reviewed and published here2.
Materials and Methods
The MASTER Trial analyzed data from 210 adult patients 18 years of age and older scheduled for surgical procedures under general inhaled anesthesia, who were randomized to either the EtC or MC. The primary objective was to determine whether EtC was non-inferior to MC in achieving and maintaining targeted EtAA and EtO2 concentrations. Secondary objectives assessed performance as measured by response time, overshoot, and accuracy. Tertiary objectives included calculation of inhaled anesthetic agent usage. Data analysis included descriptive statistics and statistical tests to test for significance.
Results
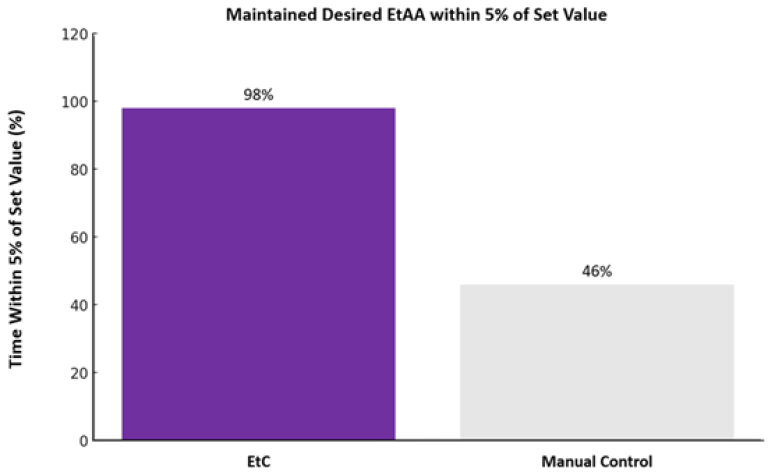
The percentage of time that EtC maintained targeted EtAA concentrations within the non-inferiority limit was significantly higher than MC. EtC maintained EtAA within 5% of the set value 98 ± 2% of the time, compared to 46 ± 32% of the time with MC (p < 0.0001). See Figure 1.
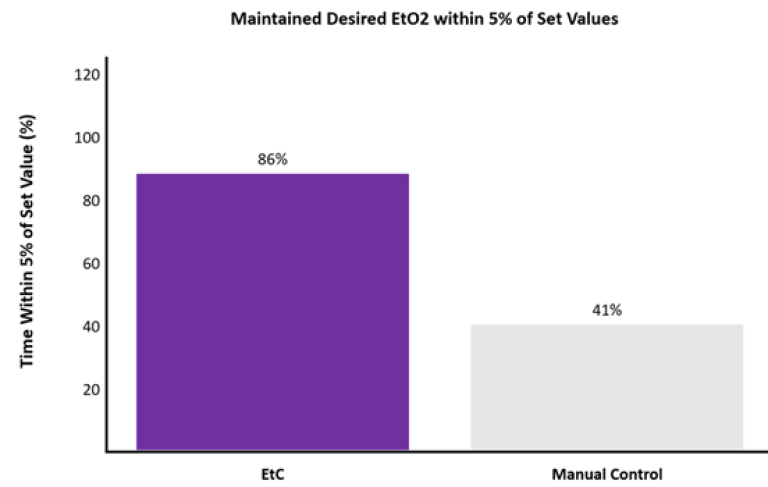
Moreover, EtC maintained EtO2 within 5% of the set values 86 ± 23% of the time compared to 41 ± 33% of the time with MC (p < 0.0001). See Figure 2.
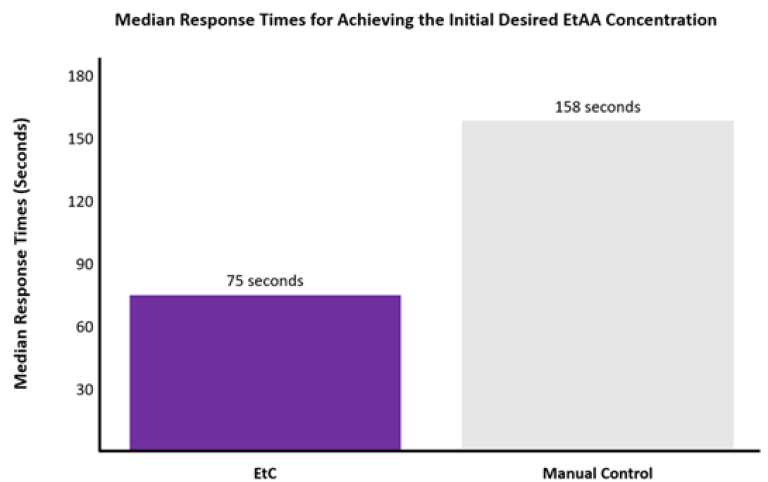
Response times for achieving 90% of the initial desired EtAA concentration were significantly faster with EtC (median 75 seconds) compared to MC (median 158 seconds) (p=0.0013). See Figure 3.
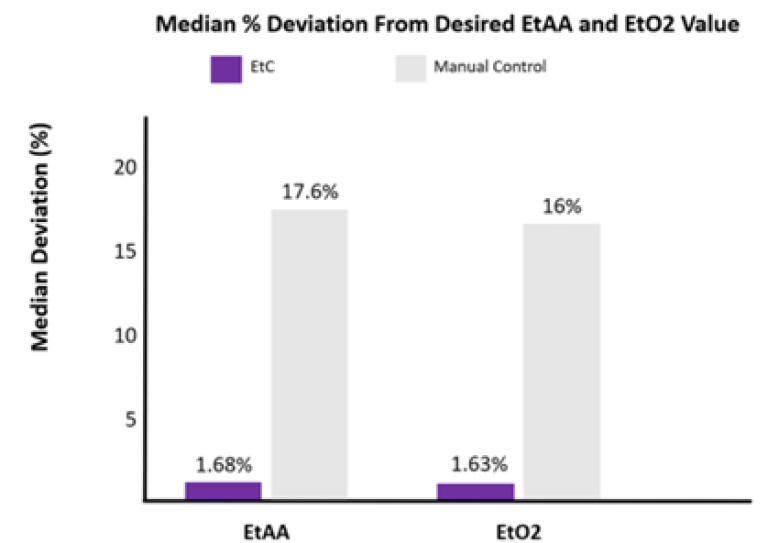
Additionally, EtC resulted in a lower median percent deviation from the clinician’s desired EtAA and EtO2 concentrations than MC. Median percent deviation from the clinician’s desired EtAA and EtO2 with EtC was 1.68% and 1.63% respectively, whereas with the MC, deviation was 17.6% and 16% respectively. See Figure 4.
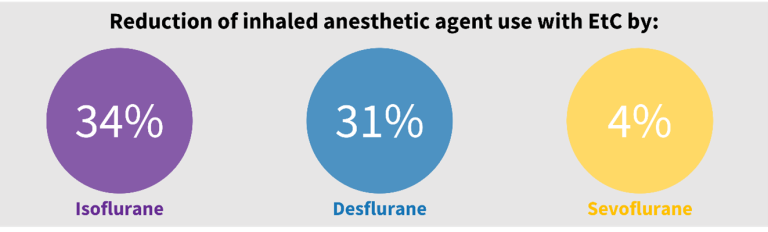
EtC basal fresh gas flows were set to 0.5 liters/minute for desflurane and isoflurane and 2 liters/minute for sevoflurane. Resultant anesthetic agent usage was reduced, particularly for isoflurane (34%, p<0.001) and desflurane (31%, p=0.025), compared to MC. Vasoactive medication administration and adverse events were comparable between groups, with no safety concerns identified with EtC use.
Importantly, EtC exhibited a median overshoot (amount the controller or vaporizer delivery exceeded the desired EtAA) of 6.64% of the selected EtAA concentration, whereas the median overshoot with MC was 0% as most MC patients failed to reach the clinician’s desired value.
Discussion and Conclusion
The results of the MASTER Trial confirm the safety and efficacy of the EtC system in anesthesia delivery. EtC demonstrates greater accuracy, precision, efficiency, faster response times, and reduced anesthetic agent usage compared to manual control, providing significant clinical benefits. These findings underscore the potential of EtC to enhance patient care and resource utilization in surgical settings, supporting its adoption as a standard practice for anesthesia delivery.
* End-tidal Control in the United States is indicated for patients 18 years of age and older.
References
1. Karen C. Nanji, Amit Patel, Sofia Shaikh, Diane L. Seger, David W. Bates; Evaluation of Perioperative Medication Errors and Adverse Drug Events. Anesthesiology 2016; 124:25–34 doi: https://doi.org/10.1097/ALN.0000000000000904
2. McCabe, Melissa D. MD, FASA, MSCR*; Dear, Guy de L. MB, FRCA†; Klopman, Matthew A. MD, FASA, FASE‡; Garg, Kritika MS§; Seering, Melinda S. MD, FASA, MHCDS‖. End-Tidal Control Versus Manual Control of Inhalational Anesthesia Delivery: A Randomized Controlled Noninferiority Trial. Anesthesia & Analgesia ():10.1213/ANE.0000000000007132, July 19, 2024. | DOI: 10.1213/ANE.0000000000007132
JB29933XX
©2024 GE HealthCare
GE is a trademark of General Electric Company used under trademark license. Reproduction in any form is forbidden without prior written permission from GE HealthCare. Nothing in this material should be used to diagnose or treat any disease or condition. Readers must consult a healthcare professional.