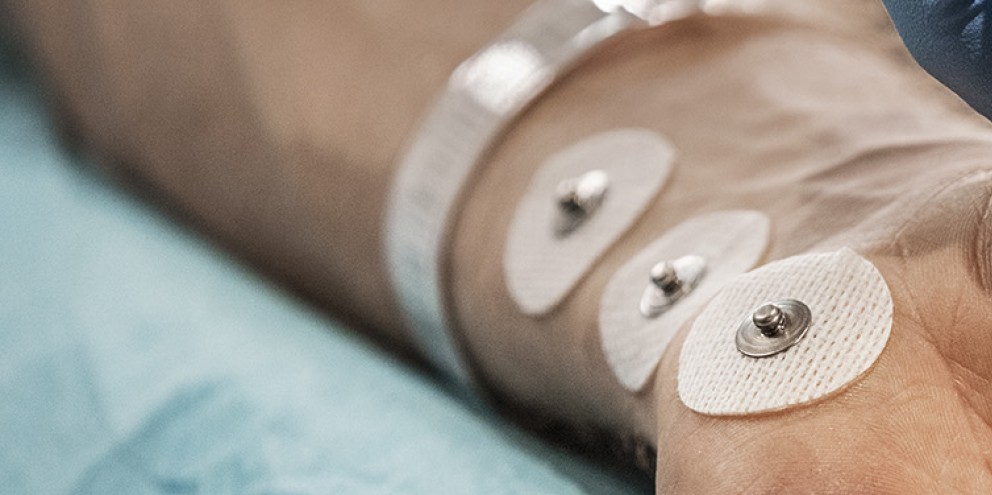
Dr. Manfred Blobner, MD, a professor and researcher at Technische Universität München in Munich, Bayern, Germany, believes that all patients receiving non-depolarizing NMBAs should be monitored by quantitative neuromuscular techniques in order to be certain that no residual neuromuscular blockade exists prior to removal of the endotracheal tube and subsequent transportation to the post-anesthesia care ward. Dr. Blobner is a leading voice in the field of neuromuscular blockade and neuromuscular monitoring having studied the field for nearly three decades.
Dr. Blobner started his investigations into neuromuscular junction physiology and monitoring in 1989 and has since conducted pivotal studies and published several peer-reviewed articles on the subject. In November of 2018 Dr. Blobner published the results of his post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR) study, which has laid the groundwork for a much deeper understanding of residual neuromuscular blockade, and its implications on outcomes following anesthesia with muscle relaxation.1
In 2018 a group of experts in the field of neuromuscular blockade monitoring issued a consensus statement in Anesthesia & Analgesia concluding that whenever a neuromuscular blocker is administered, neuromuscular function must be monitored by observing the evoked muscular response to peripheral nerve stimulation. Ideally, stimulation of the ulnar nerve should be performed and the muscle response should be measured quantitatively at the adductor pollicis muscle or the hypothenar muscles.2
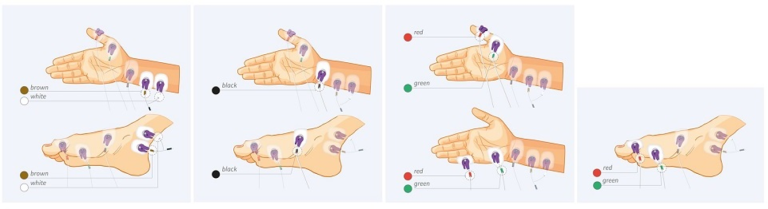
Dr. Blobner states that small degrees of neuromuscular blockade can only be accurately measured by using a quantitative monitoring device. He goes on to explain that an observer, even a highly skilled anesthesiologist, cannot reliably obtain muscular fade by tactile or visual sense when train-of-four ratio (TOFR) is between 0.4 and 0.9. The most commonly used quantitative neuromuscular monitoring techniques include acceleromyography (AMG), kinemyography (KMG) and electromyography (EMG).
AMG measures the evoked muscle contraction via acceleration of a piezoelectric ceramic wafer while KMG converts physical motion into electric current by deformation of a piezoelectric film. EMG on the other hand, measures the compound muscle action potential, which is the first signal we are able to measure after neuromuscular transmission.
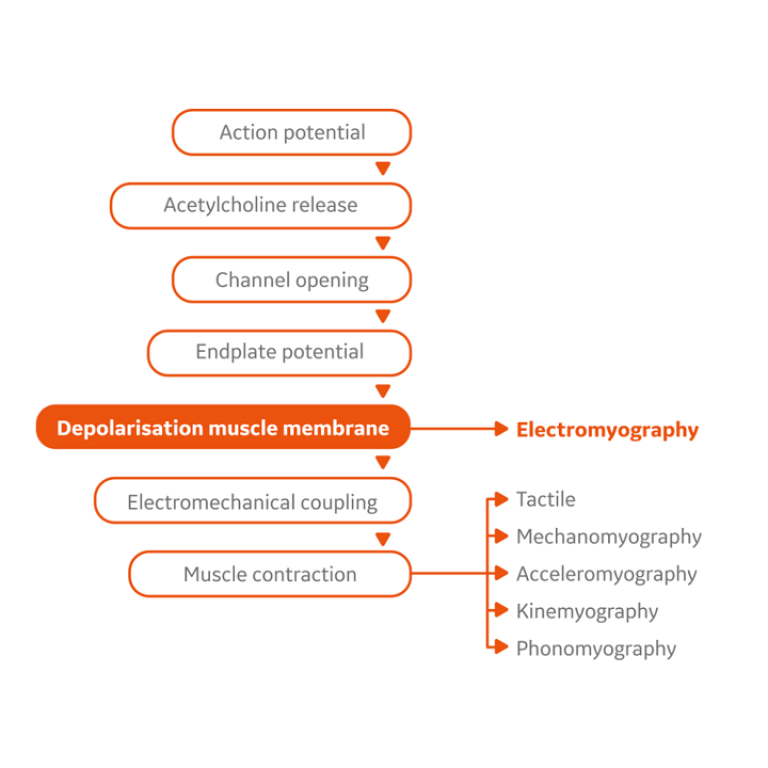
Therefore, EMG is most specifically suited to monitor the effect of muscle relaxants without any mechanic interferences, in particular it most specifically identifies any residual neuromuscular block. Further, Salminen et al. found that KMG and AMG tend to overestimate the degree of neuromuscular recovery when compared to EMG showing that EMG is more likely to reveal residual neuromuscular blockade than other technologies.3
With patient outcomes paramount on the minds of clinicians Dr. Blobner advocates for quantitative neuromuscular monitoring preferably electromyography when neuromuscular blockers are used citing the benefits, confirmed in the literature, as reducing PACU length of stay by as much as 80 minutes4, and facilitating the appropriate dosing of both neuromuscular blocking agents and reversal agents5,6. Further evidence suggest that the use of quantitative neuromuscular monitoring may reduce acute respiratory events, muscle weakness, and delays in extubation.7 Dr. Blobner agrees that significant cost reductions could result from appropriate drug dosing and most certainly with shorter postoperative stays and fewer pulmonary complications.
While the evidence is clear that quantitative neuromuscular monitoring is mandatory for detecting residual neuromuscular blockade after muscle relaxation for surgery, which method is the gold standard? Dr. Blobner points out that EMG has been recognized as the currently available gold standard due to its higher accuracy, lower sensitivity to changes in muscle contractility, independence of the hand position, the free mobility of the thumb, and any preload2.
Currently, GE Healthcare is the only manufacturer of a commercially available EMG device. Its E-NMT module provides quantitative neuromuscular monitoring displayed on a multi-parameter monitor. From an ergonometric perspective, the modular design allows the anesthesiologists’ attention on all vital parameters with one view. The E-NMT module allows for automatic cycling at a user defined time interval each time providing three parameters: Train-of-Four ratio, Train-of-Four count, and post-tetanic twitch count. Therefore, the E-NMT module provides quantitative neuromuscular monitoring in the widest available range of neuromuscular blocks2.
With the introduction and widespread use of the neuromuscular blockade reversal agent Sugammadex, Dr. Blobner was asked whether he thought this safe and extremely effective but very expensive agent had any effect on the utility of quantitative neuromuscular monitoring. In response, he noted that the dosing practices of anesthesia clinicians vary widely with regards to Sugammadex for reversal of neuromuscular blockade. Unfortunately, the manufacturer does not label doses of Sugammadex suitable to reverse the most frequently observed residual neuromuscular blocks of TOFR between 0.2 and 0.9, which can be effectively reversed by lower doses based on dose-finding studies only. Therefore, anesthetists tend to use Sugammadex in an off-label fashion to save money. Dr. Blobner does, of course, not recommend such practice. However, recognizing this approach as clinical reality, he strongly recommends to carefully monitor complete neuromuscular recovery with stable TOFR > 0.9. Since EMG does not overestimate the TOFR, it is the preferable technique to guarantee complete and stable recovery. Future research looking at quantitative neuromuscular monitoring and titration of both blocking agents and reversal agents has to proof safety and cost efficacy of this approach.
Embracing new techniques and technology to a practice is not always easy. Although not a new technology, the use of EMG and quantitative neuromuscular monitoring in general are not consistent or widespread among anesthesia practitioners. Dr. Blobner addressed this in a recent interview citing entrenched providers as a major obstacle to embracing this technology. He went on to offer a roadmap for more widespread and even universal adoption of quantitative neuromuscular monitoring. First, Dr. Blobner considers it crucial that hospitals and departments of anesthesia provide quantitative neuromuscular monitoring at every site where anesthesia is given. Secondly, he suggests that education be disseminated on the benefits of quantitative neuromuscular monitoring on patient outcomes, costs, and quality of care. Finally, Dr. Blobner calls on the various governing bodies involved in producing guidelines for best anesthesia practices to examine the evidence and issue a call to include quantitative neuromuscular monitoring as a mandatory or standard monitor in all delivered anesthetics requiring neuromuscular blockade.
Based on the evidence and Dr. Blobner’s thoughtful reflections on quantitative neuromuscular monitoring, clinicians should take a closer look at how they provide the best care for their patients undergoing neuromuscular blockade. The benefits are clear and may include avoidance of serious post-operative complications, shorter stays, and lower costs. As scientists/clinicians, we trust quantitative data as a matter of course as it is reproducible, easily studied, and more accurate than observational, qualitative measures. Just as it is clear that quantitative neuromuscular monitoring is superior to qualitative monitoring, EMG represents a more accurate, less complicated, and easier to use platform when compared to acceleromyography. We look forward to Dr. Blobner’s continued efforts to champion and realize the true potential of this important technology.
References:
- Kirmeier E, Eriksson LI, Lewald H, Jonsson Fagerlund M, Hoeft A, Hollmann M, Meistelman C, Hunter JM, Ulm K, Blobner M; POPULAR Contributors.Post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR): a multicentre, prospective observational study. Lancet Respir Med. 2019 Feb;7(2):129-140. doi: 10.1016/S2213-2600(18)30294-7. Epub 2018 Sep 14. Erratum in: Lancet Respir Med. 2018 Nov 6
- Naguib, M, Brull S, Kopman A, et al. Consensus statement on perioperative use of neuromuscular monitoring. Anesthesia & Analgesia. 127(1):71–80, JUL 2018 DOI: 10.1213/ANE.0000000000002670
- Salminen J., et al. Comparison of train-of-four ratios measured with Datex-Ohmeda’s M-NMT MechanoSensor™ and M-NMT ElectroSensor™. Journal of Clinical Monitoring and Computing, 30(3), 295-300 (2016).
- Butterly A, Bittner EA, George E, et al. Postoperative residual curarization from intermediate-acting neuromuscular blocking agents delays recovery room discharge. Br J Anaesth. 2010 Sep;105(3):304-9. doi: 10.1093/bja/aeq157. Epub 2010 Jun 24.
- Miller RD, Ward TA. Monitoring and pharmacologic reversal of a nondepolarizingneuromuscular blockade should be routine. Anesth Analg. 2010 Jul;111(1):3-5. doi: 10.1213/ANE.0b013e3181e13522.
- Kaufhold N., et al. Sugammadex and neostigmine dose-finding study for reversal of residual neuromuscular block at a train-of-four ratio of 0.2 (SUNDRO20). British Journal of Anaesthesia, 116(2), 233-240 (2016).
- Murphy G.S. and Brull S.J., Residual neuromuscular block: lessons unlearned. Part I: definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesthesia & Analgesia, 111(1), 120-128, (2010).
Professor Manfred Blobner is Senior Consultant and Vice Director of the Department of Anaesthesiology and Intensive Care Medicine, School of Medicine, Technical University of Munich. During the last 25 years at the department he inaugurated and chaired seven clinical and experimental research groups which became independent under his former assistants and followers and are still working in their respective fields successfully. He still keeps the lead of the research group on Neuromuscular Physiology and Pharmacology. He is author of 152 manuscripts published in international journals (WOS h-index is 27). He is chief investigator of ESA CTN’s POPULAR study and member of ESA’s scientific subcommittee of Pharmacology.

Prof. Manfred Blobner
Prof. Blobner is a Professor of Anesthesiology and the Coordinator of the Residency Program at Technische Universität München. He completed his Medical degree from the Faculty of Medicine of Technische Universität München. He also completed his training from the Department of Anesthesiology of the same institution. He holds Diplomas in Emergency Medicine, Anesthesiology, and Intensive Care Medicine. He has won multiple Excellence in Research Awards from different medical societies. He has been the Principal Investigator in multiple clinical trials. He has authored 136 journal publications, written 10 book chapters, and is a reviewer for 9 international medical journals. His clinical fields of interest include: outcomes related to perioperative volume resuscitation techniques, outcomes related to management of neuromuscular function, and early goal-directed mobilization.