Why is quantitative neuromuscular monitoring (NMT) so important when administering NMBAs?
Ms. Harris: The most important reason is there is no other way to ensure full neuromuscular recovery without quantitative monitors. Many clinicians are unaware of the limitations of qualitative assessment and have a false sense of assurance of what a peripheral nerve stimulator can tell you. There’s also a false assurance of what the physical signs of recovery indicate in terms of the degree of recovery from NMBA.
Until you routinely use a quantitative NMT monitor you don’t know what you don’t know. The evidence is clear that quantitative monitoring should be the gold standard. Currently, that’s not the case yet in the U.S. as far as making quantitative NMT monitoring part of a standard of care like it is in some European countries. However, this is likely to rapidly change as the ASA just published a report by the Task Force on Neuromuscular Blockade providing Practice Guidelines for Monitoring and Antagonism of Neuromuscular Blockade.
When you were in training to become a CRNA, did you receive quantitative NMT training, specifically EMG?
Ms. Harris: I did my anesthesia training in 2010-2013 and at that time none of the hospitals I rotated to for my clinicals had a quantitative monitor available. Only after I completed my thesis project on neuromuscular blockade did I start to have an interest in using them.
[During my research] I saw a huge variation in practice and usually when there is huge variation in practice regarding choosing neuromuscular blocking agents and how and when they choose to reverse – that’s usually a sign something’s not right.
This led me to investigating further and becoming fully convinced that quantitative monitoring should be required in all anesthetics where a neuromuscular blocking agent (NMBA) is being administered.
At this point, I knew everything there was to know about quantitative monitoring but had yet to actually use one until I started working at Duke. There were monitors in every room, but no one knew what they were, nor did they use them.
That is what started me down the path to determine why did we have this technology if we weren’t using it and how we could start implementing it.
Why did your department decide to implement quantitative NMT monitoring?
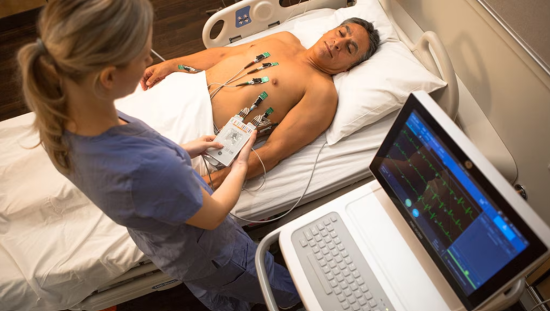
When we first started looking into quantitative monitoring, it took me two years of routinely using an electromyography (EMG) quantitative monitor on all of my patients to be able to demonstrate to my colleagues the benefits of using it. I would often use the NMT on my colleagues’ patients who were having issues after extubation.
The EMG monitor would frequently show why that patient was struggling – often because they were still partially paralyzed.
This plus a series of events of having the right anesthestiologist in the right place who was also convinced of the importance of quantitative monitoring led to the start of planning for department implementation.
We started a six-month effort to implement – our goal was to have quantitative EMG monitoring in place by the start of the next residency program.
We worked with a CRNA who wanted to perform a QI project to evaluate residual neuromuscular blockade in the post anesthesia care unit (PACU). We evaluated patients on arrival to PACU using portable EMG monitors to identify the incidence of residual neuromuscular blockade. We found very quickly within the first week a significant percentage of patients were arriving to the PACU with residual neuromuscular blockade.
Around this same time, the new reversal drug Sugammadex had become available for use. Even though our Sugammadex use quadrupled that week, we still observed cases of residual paralysis in PACU. This highlighted where we were falling short and helped everyone see this was happening – despite the use of Sugammadex.
An added benefit of Sugammadex use was, due to its rapid onset, you could observe an immediate improvement in the patient’s clinical symptoms of residual neuromuscular blockade along with the corresponding improvement in the TOF ratio measured by EMG. For the first time, clinicians could quantify the clinical assessment findings of residual neuromuscular blockade.
How was your initial experience and what barriers did you encounter implementing EMG monitoring in the clinical setting?
The hardest barrier was convincing people of the need to use them. Once you convinced them of the patient care benefit, the challenge became overcoming the huge change in practice.
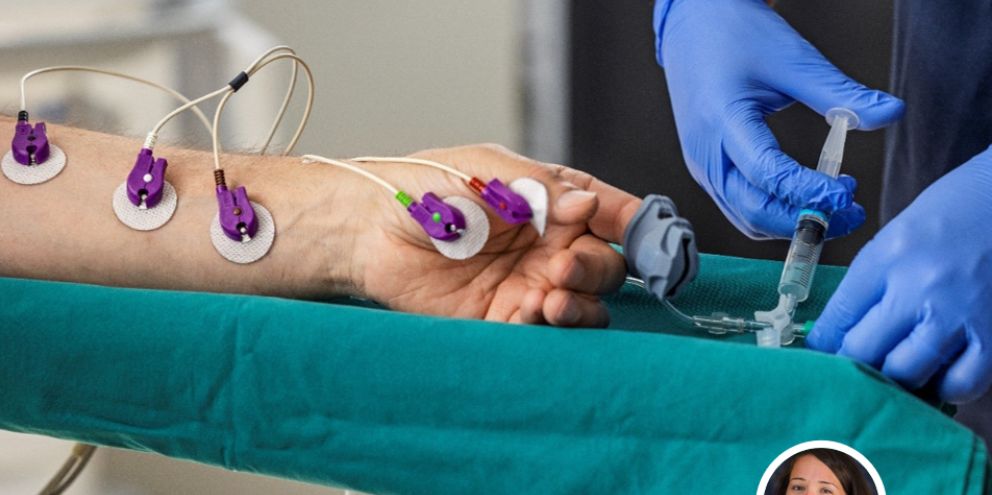
EMG monitoring is another monitor to apply and it’s difficult to apply because electrode placement is important. Other monitors like ECG leads can be placed suboptimally and you still get a good signal, but with EMG electrodes, poor placement can really impact readings.
The electrodes need to be applied prior to the induction of anesthesia so implementing EMG monitoring is also altering the entire anesthesia practice and changes the flow of care for clinicians.
Another initial challenge was EMG monitoring is a new way of looking at neuromuscular blockade as you can now quantify things like a recovery response curve that you were previously unable to quantify. Because of this, there were many nuances in understanding the physiologic signal users were looking at and a learning curve for interpreting the monitored data.
Our adherence was a one-to-two-year process to get things fully on board. People would try using the monitors at first and then fail - often due to user error - and then give up and stop using it. So, it was a lot of trial and error to increase adherence.
Since you recently changed practice to EMG monitoring, what did your training consist of?
We had some didactic and grand rounds with several staff meetings consisting of the monitor overview and big picture. Then me and my physician anesthesiology colleague essentially became departmental champions and would go into the operating rooms (OR) and pre-op and put the monitors on patients.
In July when new physician residents started, I would help them and teach them how to apply the electrodes correctly, how to evaluate the physiologic signal (EMG waveform) and interpret the monitored data.
I also did onboarding and direct hands-on training with all new CRNAs.
Were there logistical – for example cumbersome technology, not enough monitors for every patient, etc. – barriers to implementation?
Not really, because before we did our go-live, we had already surveyed all of the ORs to make sure they had monitors, including the pediatric ORs. It wasn’t until we implemented the protocol that people started saying monitors were broken because they weren’t working well and throwing them away. But even now, most if not all our ORs have monitors.
Is there general feedback from clinicians and clinical teams regarding EMG monitoring solutions and protocol adoption?
Most people who weren’t receptive to adding a monitor quickly changed their mind when the monitor caught something they would have originally overlooked. Things like a longer recovery period or even someone who didn’t have an expected response to Sugammadex.
It took a series of patient events that led late adopters to adopt practice.
There are also a few technical issues that have come up that have led clinicians to question if they can trust the monitors. For example, there’s not a range of reliable values that it accepts. In order to properly troubleshoot, it is critical to evaluate the actual EMG waveform. You can’t look at these monitors, get a number and be good - you have to look at the signal and interpret it further in light of the trended EMG data.
Have you noticed an improvement in reversal strategies since implementing EMG monitoring solutions?
Absolutely, I think people are far more aware of the limitations of clinical signs of recovery from neuromuscular blockade and I see people reversing earlier and not waiting until the last minute once anesthesia is already wearing off. Not every patient responds at the same time when reversing. We have far fewer providers who will reverse based on a facial nerve response because you can now see the wide variability between recovery of the facial muscles versus peripheral muscles.
How can we improve EMG monitoring protocol adherence in the clinical setting?
You need content experts in the health system who have spent the time with the monitor to fully understand it. Another way is to educate on the FDA approval process for pediatric versus adult use. For example, some clinicians, specifically in the pediatric space, are unwilling to implement it because they believe that NMT is not FDA approved for use in pediatric patients.
Have you used analytics to show improved outcomes to clinical teams?
All we did early on was look at the reintubations we had in a year. We averaged one or two per month and observed a decrease to six per year within the first year after implementation. This implication is huge because the recovery room will not take intubated patients so that’s an intensive care unit bed being used by a patient which can cause a huge strain on the system.
But there are so many other variables that factor into this number to fully say the use of quantitative monitoring was responsible for the trend. Otherwise, we don’t have further studies right now.
Can you have financial savings by using quantitative monitoring?
Thanks to the monitoring, you can use significantly less Sugammadex. With the introduction of Sugammadex people saw it as the magic drug and if you use it, you don’t need any monitoring at all.
But that’s not the case, the dosage of the drug can change and how the patient responds can change. Rather than using entire vials, our pharmacy makes smaller 200mg syringes of Sugammadex that has contributed to significant cost savings. The only way we can use this smaller dosage is through routine use of quantitative monitoring.

Erica Harris CRNA
Erica M Harris is a Certified Registered Nurse Anesthetist (CRNA) Specialist in Durham, North Carolina. She graduated with honors in 2013. Having more than 10 years of diverse experiences, especially as a Certified Registered Nurse Anesthetists (CRNA), Erica M Harris affiliates with Duke University Hospital, cooperates with many other doctors and specialists in medical group Duke University Health System Inc.