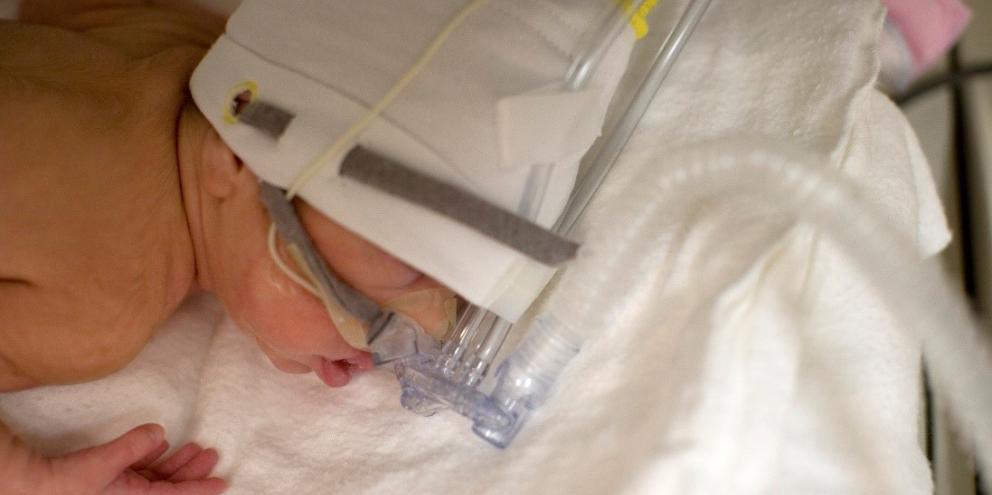
Invasive support can not only lead to inflammation of the lungs in the short-term, it can also result in impaired development and scarring of the lungs in the long-term2. Because of this, the condition, known as bronchopulmonary dysplasia (BPD), is widely acknowledged as one of the major morbidities among preterm infants5. It’s a condition that is associated with adverse pulmonary and neurological outcomes5.
That’s why in an effort to decrease the risks of BPD and provide the highest level of care for these vulnerable patients, non-invasive ventilation (NIV) has emerged as the treatment of choice for providing positive pressure ventilatory support at the time of delivery or during the transitional period3.
In this article, we’ll review current NIV support strategies, including high flow nasal oxygen (HFNO)*, nasal continuous positive airway pressure (nCPAP) and nasal intermittent positive pressure ventilation (NIPPV) and discuss the benefits of each in the respiratory management of preterm infants.
HFNO – Reducing Nasal Injury
HFNO utilizes a nasal cannula to provide inhaled gases at flows above the inspiratory demand flow3. This results in washout of nasopharyngeal dead space and reduced inspiratory resistance, while decreasing the work of breathing3,5. Benefits offered by HFNO include ease of use, less risk of nasal injury and improved comfort for the infant3.
A systematic review and network meta‐analysis by Ramaswamy et al. found that HFNO was equally efficacious as CPAP for infants with RDS, findings which were confirmed by Hong et al and Fleeman et al1.
The 2016 Cochrane review comparing HFNO to CPAP also found no difference in the rate of treatment failure or reintubation within 7 days5. Yet, the majority of the studies included infants greater than 28 weeks’ gestation5. Therefore, these findings might not be generalizable to infants below that mark, making drawing accurate conclusions challenging.
However, this form of NIV is not without its drawbacks. Because the delivery of HFNO relies on a loose-fitting nasal prong, pressure delivery is not reliable and is neither measured nor controlled by the clinician3,5.
Additionally, clinical evidence is uncertain regarding efficacy and risk of treatment failure in HFNO compared to other respiratory support measures, including CPAP.
To add more uncertainty, one of the largest retrospective studies of 2,487 extremely preterm infants, Taha et al found that HFNO resulted in an increased risk of death or BPD, as well as longer length of stay compared to nCPAP5.
nCPAP – Limiting the needs for IMV and Surfactant Administration
nCPAP delivers nasopharyngeal pressure, which is maintained during both inspiration and expiration, to a spontaneously breathing infant. Because of this, nCPAP reduces airway resistance, boosts functional residual capacity. It also works to stabilize the chest wall and splint the upper and lower airway3.
This NIV option also helps to preserve endogenous surfactant and decrease the need for additional surfactant administration3. This may decrease the need for and length of intubation and IMV, as well as provide added protection against BPD3,5.
In fact, Anne and Murki’s review of evidence found that in neonates with RDS, nCPAP reduces surfactant administration and the need for IMV3. This evidence was compounded by Rüegger et al, who found that prophylactic nCPAP, lowers the rates of both BPD alone and the combined outcome of death or BPD, when compared with immediate endotracheal ventilation2.
As a result, augmenting nCPAP with NIPPV may help reduce adverse outcomes, improve ventilation, and decrease the need for intubation2,5.
Yet, as with HFNO, nCPAP offers a number of downsides in addition to its clinical benefits.
As Shi et al point out, nCPAP often fails in infants with hypopnea or apnea, requiring intubation and IMV5. CPAP failure rates remain at approximately 50% in the first week of life in extremely preterm infants – those who have the greatest risk of developing BPD2.
NIPPV – Lowering Risk of Respiratory Failure
NIPPV combines a continuous positive end-expiratory airway pressure (PEEP) with intermittent higher pressures, delivered by a nasal mask or nasal prongs. It can rely on one of two types of devices – ventilators or flow-drivers, which may offer the option to synchronize pressure changes with spontaneous breathing2.
NIPPV offers all of the benefits of nCPAP, along with added positives. The non-invasive option further reduces upper airway resistance, enhances spontaneous inspiratory effort, and improves compliance and alveolar recruitment, while reducing chest wall distortion5.
Despite the fact that there were initial concerns of increased gastrointestinal side effects with the treatment, two systematic reviews of the Cochrane Collaboration on NIPPV reported no significant differences between the NIPPV and CPAP groups in rates of feeding intolerance, gastrointestinal perforation, necrotizing enterocolitis or air leak2.
NIPPV was also found to have lesser treatment failures and reduced need for MV compared to HFNC in Anne and Murki’s review. Additionally, synchronized NIPPV (sNIPPV) was found in a randomized controlled trial comparing sNIPPV with nCPAP in preterm neonates with RDS, to lower rates of respiratory support failure, hypercarbia, and hypoxia and decrease the work of breathing3.
Rüegger et al’s meta-analysis “Nasal Intermittent Positive Pressure Ventilation for Neonatal Respiratory Distress Syndrome” pooled data from 18 trials and 1,900 infants demonstrated a clinically important, 37% relative reduction in the risk of respiratory failure with NIPPV. The results were most obvious in trials utilizing ventilator-generated NIPPV and synchronization2.
Overall, the researchers found that infants extubated to NIPPV had a 22% relative risk reduction for respiratory failure within the first week post-extubation compared with those managed with CPAP2.
These results make a case for NIPPV being the most effective primary NIV support strategy to reduce the risk of respiratory failure in the first few days of life.
Conclusion
Minimizing the duration of IMV may improve outcomes in pre-term and full-term infants with RDS. While HFNO and nCPAP support these improved outcomes, NIPPV can augment the benefits they provide, minimizing lung injury and the risk of BPD.
References
1: Ramaswamy et al. “Efficacy of noninvasive respiratory support modes for primary respiratory support in preterm neonates with respiratory distress syndrome: Systematic review and network meta‐analysis.” Pediatric Pulmonology. 2020. 55:2940–2963.
2: Rüegger et al. “Nasal Intermittent Positive Pressure Ventilation for Neonatal Respiratory Distress Syndrome.” Clin Perinatol 48 (2021) 725–744.
3: Anne, Rajendra Prasad and Murki, Srinivas. “Noninvasive Respiratory Support in Neonates: A Review of Current Evidence and Practices.” Indian Journal of Pediatrics 88(7):–(July 2021) 670678.
4: Paoli et al. “Nasal CPAP for neonates: what do we know in 2003?” ArchDis Child Fetal Neonatal Ed. 2003: F168 – F172.
5: Shi et al. “A Review on Non-invasive Respiratory Support for Management of Respiratory Distress in Extremely Preterm Infants.” Frontiers in Pediatrics. May 2020. Vol. 8: Article 270.
*In the United States the O2 Therapy mode for performing high flow nasal oxygen (HFNO) delivery on the CARESCAPE™ R860 Ventilator is intended to be used for all adult patients and pediatric patients greater than 10 kg in weight. O2 Therapy mode in the United States is not available for the neonatal patient type.
© GE, 2022 – All rights reserved.
GE, CARESCAPE and the GE Monogram are trademarks of GE. Reproduction in any form is forbidden without prior written permission from GE. Nothing in this material should be used to diagnose or treat any disease or condition. Readers must consult a healthcare professional.
JB20256XX