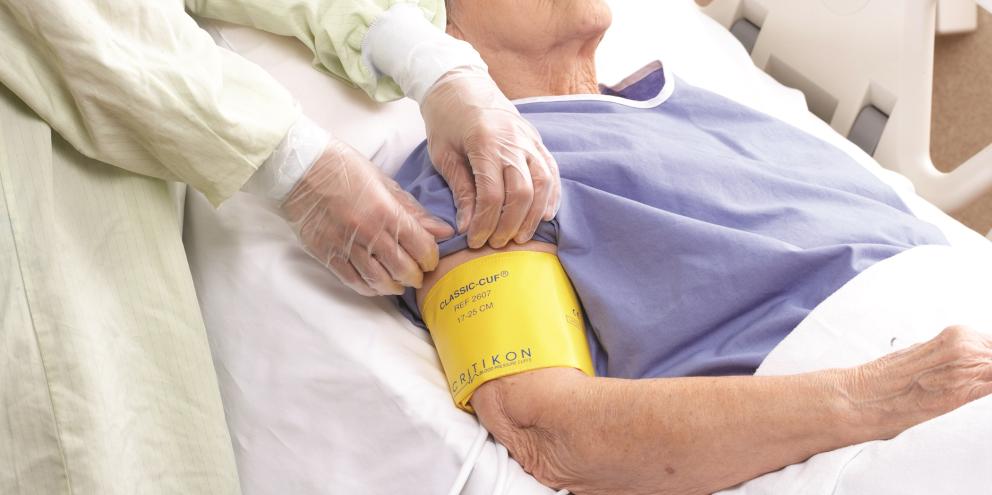
There are many causes of HAIs, including catheters, surgery, injections, improperly disinfected facilities, the transmission of communicable diseases between patients and health care workers, and overuse or improper use of antibiotics.2 The adoption of single-patient-use (SPU) blood-pressure (BP) cuffs could effectively eliminate one common source of contamination. Though the direct cost of SPU cuffs may be more than that of reusable cuffs, the extra cost is offset by the savings achieved through reducing the risk of potential infections, as explained in a white paper by Bruce Friedman.3
Impact of Blood Pressure Cuffs on HAIs
BP cuffs are one of the most frequently used medical devices, yet they are routinely ignored or inadequately handled when it comes to cleaning.4,5 Contamination among patients with pathogens cultured from BP cuffs has occurred even following disinfection of said equipment.5
Many studies have demonstrated the presence of contaminants on BP cuffs, including Clostridium difficile,6 methicillin-resistant Staphylococcus aureus (MRSA),7–10 Acinetobacter baumannii,9,11 and Escherichia coli and pseudomonas.7 Bacteria such as MRSA and vancomycin-resistant enterococci (VRE) in particular can remain viable for days on BP cuffs and other surfaces.8,12
BP cuffs are increasingly being recognized as potential vectors for HAIs. Clinical guidance from the 2008 Massachusetts Department of Public Health recommends that disposable BP cuffs be used in acute-care hospitals.13
Similarly, the Society for Healthcare Epidemiology of America guidelines for preventing the transmission of MRSA and VRE highlight that shared patient equipment, including BP cuffs, can transmit infections between patients.14
How SPU BP cuffs decrease the risk of HAIs
Infections are known to significantly increase patient care costs, hospital stay lengths, and mortality rates.15,16 The adoption of SPU cuffs could potentially reduce the frequency of HAIs, thereby improving patient outcomes, decreasing mortality, and markedly reducing financial burden.
One method of calculating the financial benefit of a device intended to prevent or reduce adverse events (such as HAIs) involves a formula used in risk-assessment cases, which is expressed as follows in Equation 117:
- B < P × L
This formula demonstrates that the cost of precautions taken to reduce an adverse event (B) can be economically justified if it is less than the product of the probability of occurrence (P) and the magnitude (L) of the resulting harm (i.e., the cost to treat the infection).
Reports have shown that the median cost of HAIs ranges between $25,000 and $40,000.15,18,19 For the purpose of the present discussion, the low end of this range will be used for L in Equation 1.
Reported rates of HAIs range from 9.8 to 23.7 per 1,000 patient-days.20,21 To accurately determine costs for SPU cuffs, HAI rates need to be adjusted for length of stay (LOS).21 Data from the Agency for Healthcare Research and Quality indicate that the average LOS for patients in acute-care hospitals is 4.6 days.22 Based on LOS, the probability of an HAI can be calculated as follows in Equation 2:
- Rate of HAIs = 0.0098/day
- Average LOS (ALOS) = 4.6 days
- Probability of an HAI occurring in an individual patient during their stay = HAI × ALOS = 0.0098/day × 4.6 days = 0.0451
Placing these data into the risk formula (Equation 1) yields the following:
- B < P × L
- 0.0451 × $25,000
- $1,127
Even though clinicians may change gloves and/or wash hands between patients, the BP cuffs themselves are not always cleaned or are cleaned inadequately.5 Beggs et al.’s study on hand-washing considered a 10% probability of patient-to-clinician transmission as well as a similar probability that a clinician would transmit the infection to another patient,23 resulting in a transmission rate of 1%. If this rate of transmission is assumed, then the acceptable per-patient cost would be as follows in Equation 3:
- ($1,127 × 1%) = $11.27
Conclusion
Since single-use BP cuffs cost much less than the estimated $11.27 calculated in the above risk-assessment model, their use for reducing HAI risk can be clearly justified.25 Moreover, this analysis does not account for the initial purchase price of reusable cuffs, or the cost of cleaning and disinfecting them, which would provide further rationale for the role of SPU cuffs in preventing contamination.
References
- Centers for Disease Control and Infection Report, (March 2009).
- Magill, S.S., et al. Multistate point-prevalence survey of health care-associated infections. New England Journal of Medicine 370.13, 1198-1208 (2014).
- Friedman, B. Improving quality of care: justifying the cost for a single-patient-use blood pressure cuff. GE Healthcare (2018).
- Beard M.A., et al. Sphygmomanometers as a reservoir of pathogenic bacteria. Med J Aust 2 (15), 758–60 (1960).
- Base-Smith V. Nondisposable sphygmomanometer cuffs harbour frequent bacterial colonization and significant contamination by organic and inorganic matter. AANA Journal 64 (2), 141–5 (1996).
- Manian F.A., et al. Clostridium difficile contamination of blood pressure cuffs: a call for a closer look at gloving practices in the era of universal precautions. Infect Control Hosp Epidemiol 17, 180–182 (1996).
- Walker, N., et al. Blood pressure cuffs: friend or foe? J Hosp Infect 63 (2), 167–9 (2006).
- Cormican M.G., et al. The microbial flora of in-use blood pressure cuffs. Irish Journal of Medical Science 1991 160 (4), 112–13 (1991).
- de Gialluly, C., et al. Blood pressure cuff as a potential vector of pathogenic microorganisms: a prospective study in a teaching hospital. Infection Control and Hospital Epidemiology 27 (9), 940–3 (2006).
- Boyce JM. Environmental contamination makes an important contribution to hospital infection. Journal of Hospital Infection 65 (S2), 50–54 (2007).
- Bureau-Chalot F., et al. Blood pressure cuffs as potential reservoirs of extended-spectrum beta-lactamase VEB-1-producing isolates of Acinetobacter baumannii. J Hosp Infect 58 (1), 91–2 (2004).
- Kleinpell R.M., et al. Targeting Health Care-Associated Infections: Evidence-Based Strategies, In: Patient Safety and Quality An Evidence-Based Handbook for Nurses. Rockville (MD): Agency for Healthcare Research and Quality (US); (2008). [Online] https://www.ncbi.nlm.nih.gov/pubmed/21328752. Accessed 28 April 2019.
- Contact precautions in hospitals. In: Prevention and control of healthcare-associated infections in Massachusetts. Part 1: final recommendations of the Expert Panel. Boston (MA): Massachusetts Department of Public Health; (2008 Jan 31.) 50-3. [Online] http://data.patientcarelink.org/uploadDocs/1/Betsy-Leham.pdf. Accessed 28 April 2019.
- Muto, C.A., et al. SHEA Guideline for Preventing Nosocomial Transmission of Multidrug-Resistant Strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol 24, 362–386 (2003).
- Zhan, C. and Miller, M. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA 290 (14), 1868–1874 (2006).
- Laupland K.B., et al. Cost of intensive care unit-acquired bloodstream infections. Journal of Hospital Infection 63, 124–132 (2006).
- Hur, D. and Gravestein J.S. Is ECG monitoring in the OR cost effective? Biotelemetry, Patient Monitoring, 6, 20020–6 (1979).
- Stone P.W. Systematic review of economic analyses of health care-associated infections. American Journal of Infection Control 33 (9), 504 (2005).
- Zhan, C. and Miller, M. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA 290 (14), 1868–1874 (2006).
- Elward A.M., et al. Attributable cost of nosocomial primary bloodstream infection in pediatric intensive care unit oatients. Pediatrics, Apr, 115, 868–872 (2005).
- Jarvis W.R. Nosocomial infection rates in adult and pediatric intensive care units in the United States.National Nosocomial Infections Surveillance System. Am J Med, 91 (3B), 185S–191S (1991).
- Weinstein R.A., Nosocomial infection update. Emerg Infect Dis 4, 3, 416-20 (1998).
- AHRQ, Center for Delivery, Organization and Markets, Healthcare Cost and Utilization Project, Nationwide Inpatient Sample (1993–2005). [Online] http://www.hcup-us.ahrq.gov/reports/factsandfigures/figures/2005/2005_1…. Accessed 28 April 2019.
- Beggs C.B., et al. Increasing the frequency of hand washing by healthcare workers does not lead to commensurate reductions in staphylococcal infection in a hospital ward BMC Infectious Diseases, 8:114 (2008). [Online] http://www.biomedcentral.com/1471-2334/8/114. Accessed 28 April 2019.
- Alexander, E.S., et al. Implementation of disposable blood pressure cuffs as a novel approach to reduce fomite transmission of healthcare-associated (HCA) Clostridium difficile infection (CDI) in a community hospital or twice implemented is once credible. American Journal of Infection Control, E61-62, June 2009.