The FDA, the International Organization for Standardization (ISO) and the medical device industry are doing what they can to help give clinicians awareness on the implications of misconnections and reduce the likelihood of these types of use errors.
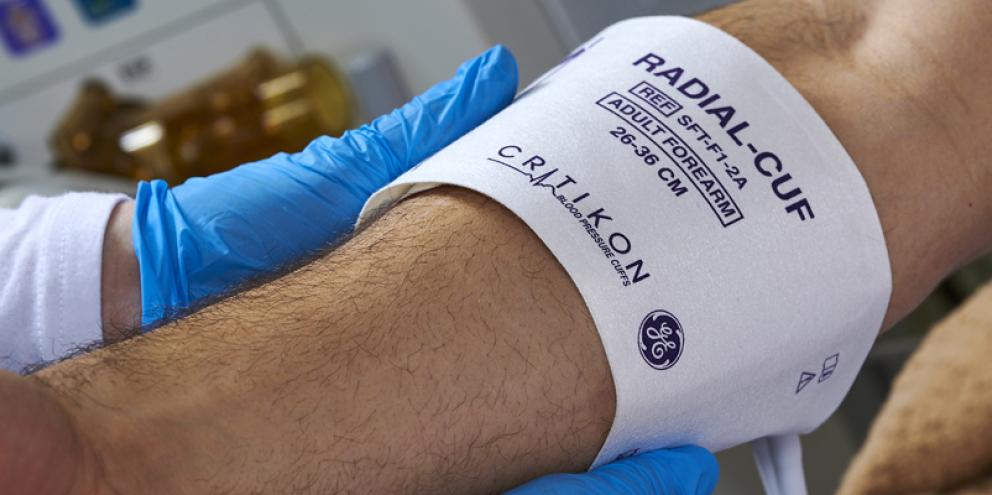
For example, GE HealthCare is strengthening its ongoing commitment to patient safety by upgrading its existing DINACLICK™ connection system for single and dual lumen non-invasive blood pressure cuffs to be compliant with the ISO-80369-5 standard. Additionally, its straightforward design helps to reduce user confusion around connections that could impact patient safety.
Changes in context
Given the potential for catastrophic consequences, in 2016, the International Organization for Standardization (ISO) proposed an updated standard—called ISO 80369-5—designed to prevent misconnections between small-bore connectors. The standard specifically recommends against the use of luer-style connectors, which a correspondence in Anesthesiology pointed out, have long been associated with concerns. This category of connectors includes screw, submin, and mated submin connectors. The new guidance favors bayonet-style connectors.
Compliant with—yet introduced prior to—the ISO-80359-5 standard and grounded in patient safety, the GE HealthCare´s DINACLICK connection system is designed to eliminate the patient risks such as air emboli or even death posed by misconnecting non-invasive blood pressure cuffs and air hoses. With an audible, tactile click that mimics haptic technology, caregivers can know and feel confident they've made a solid connection between a single or dual lumen NIBP cuff and an NIBP hose to ensure accurate blood pressure readings.
The DINACLICK connection system is available for the full range of CRITIKON™ blood pressure cuffs and air hoses in sizes ranging from infant through adult. It can work for both single-tube and dual-tube NIBP hoses. The intuitive and ergonomic design also makes the system easy to connect and disconnect in all care areas throughout the hospital, allowing cuff standardization. Standardizing your facility to the DINACLICK connection system can also help with inventory optimization.
For additional patient safety, DINACLICK connectors are not made with natural rubber latex or di(2- ethylhexyl) phthalate (DEHP) and do not include any metallic components.
To learn more about the DINACLICK connection system, watch this short video demonstrating how you can improve patient safety related to blood pressure cuffs at your own institution.